Abstract
A total of 25 specimens of Eryoneicus larvae were collected near the Balearic Archipelago (Western Mediterranean Sea) in 2009 and 2010. Detailed morphological examination indicated that the smallest individual corresponded with the first zoea (ZI) stage of Polycheles typhlops hatched from a berried female by Guerao and Abelló (J Nat Hist 30(8):1179–1184, 1996). Only two species of deep-sea polychelid lobster, namely P. typhlops and Stereomastis sculpta, are known to occur in the Mediterranean. Genetic distance comparisons and phylogenetic analysis of the mitochondrial 16S rDNA and Cox I genes of this early larva together with adults from several Polycheles and Stereomastis species allowed us to assign it to P. typhlops. This is the first wild-caught larval stage of a polychelid lobster being identified using molecular techniques. The remaining specimens were attributed to zoeal stages II–III and decapodid stage based on morphological comparison. The arrangement of spines along the anterior part of the middorsal line (R, 1, 1, 1, 2, C1), characteristic of the former species E. puritanii, discriminates these larvae from other Eryoneicus found in the Mediterranean. The clear presence of epipods on the third maxilliped and pereiopods of the decapodid stage gives further support to the identification of E. puritanii as the larval stages of P. typhlops. Additionally, information on the ecology of these larvae, their abundances during different seasons, as well as their bathymetric distribution is reported.
Similar content being viewed by others
Introduction
Mediterranean deep-sea benthos is dominated by fishes and decapod crustaceans, but the biology of several key groups is still largely unknown (Cartes and Abelló 1992). Polychelid lobsters are often referred to as “deep-sea blind lobsters” because all extant forms live in deep water and have reduced eyes. These lobsters can be easily distinguished from other reptantia decapods by the presence of well-developed chelae on pereiopods 1–4 (Galil 2000; Ahyong 2009). The Polychelidae now includes over 40 extant and fossil species, but the systematics of the family is still under debate, especially at the genus level (Ahyong and Brown 2002; Ahyong and Chan 2004; Ahyong and Galil 2006). Despite several taxonomic uncertainties have been clarified within this family in recent years (Ahyong 2009; Chan 2010), studies concerning the biology of polychelids are still lacking. There is some information available on the trophic role of these lobsters (Cartes 1993, 1998; Maynou and Cartes 1998; Cartes et al. 2007; Gastoni et al. 2010), but sampling difficulties have prevented scientists from understanding the ecology of polychelids in detail. Knowledge of the reproductive biology of deep-sea species is also scarce and mostly confined to a few species from deep continental slopes and hydrothermal vent habitats (Wenner 1979; Abelló and Cartes 1992; Mullineaux et al. 1995; Maiorano et al. 1998; Company and Sardà 1998; Company et al. 2003; Follesa et al. 2007; Cabiddu et al. 2008). Similarly, the links that have been established to date between deep-sea benthic adults and pelagic larval forms are uncertain.
Only two species of polychelid lobsters, namely Polycheles typhlops (Heller 1862) and Stereomastis sculpta (Smith 1880), are known to occur in deep-sea muddy sand bottoms of the Mediterranean Sea (Zariquiey-Alvarez 1968). Previous studies carried out in NW Mediterranean have reported P. typhlops at depths between 300 and 2,000 m and S. sculpta between 1,196 and 2,261 m (Abelló and Cartes 1992; Cartes et al. 1993; Company and Sardà 2000; Company et al. 2003, 2004; Follesa et al. 2007). Females of both species can be found in shallower depths than males, suggesting a relationship with reproductive behaviour (Abelló and Cartes 1992). In both P. typhlops and S. sculpta, females attain larger sizes than males, with the largest females being more likely to be found gravid than smaller females. Ovigerous females of P. typhlops have been captured throughout the year in the Western Mediterranean (Abelló and Valladares 1988; Maiorano et al. 1998; Company et al. 2003; Follesa et al. 2007). Gravid females of Stereomastis nana from the western north Atlantic were also caught throughout the year and presented a size-distribution pattern similar to local populations of P. typhlops (Wenner 1979). Spawning-related movements to locate the optimal depth range for hatching may explain the large proportion of ovigerous females at the shallowest depths of the species distribution, as found in other bathyal species (Wigley et al. 1975; Somerton 1981; Abelló and Macpherson 1991). Knowledge on larval morphology, vertical distribution and ecology of many decapod species is still scarce in the Western Mediterranean (Torres et al. 2013, 2014) and particularly for deep-sea species. This is largely due to the relative scarcity of plankton samples covering the entire water column and the lack of expertise for their correct identification. Moreover, the capture of living deep-sea lobsters and their larval-rearing under laboratory conditions presents serious difficulties.
Several planktonic specimens collected from Mediterranean or nearby Atlantic waters, and originally described under the generic name Eryoneicus, have been claimed to correspond to the larval stages of Polychelidae (Bernard 1953; Fredj and Laubier 1985). The first description for the genus Eryoneicus, a specimen “half an inch” long captured around 3,000 m depth in the Canary Islands, was reported as Eryoneicus caecus by Bate (1888) and later as Eryoneicus faxoni by Bouvier (1905). Several smaller specimens, ranging from 5 to 10 mm in total length, were caught in the Gulf of Napoli and described as Eryoneicus puritanii by Lo Bianco (1903). Another specimen captured around 3,000 m depth in Azores waters was named Eryoneicus spinoculatus by Bouvier (1905, 1917) and then Selbie (1914) described three new species, namely E. hibernicus, E. scharffi and E. kempi, based on late-stage larvae collected from north Atlantic waters. According to Fredj and Laubier (1985), out of the four different Eryoneicus species that can be found in deep Mediterranean Sea waters, one type (E. puritanii) could be assigned to P. typhlops, whereas the other three (E. faxoni, E. kempi and E. spinoculatus) could not be accepted as larval stages of S. sculpta and perhaps belong to adult species that still have to be discovered (Fredj and Laubier 1985). All of these late-stage Eryoneicus show a very inflated carapace with numerous spines and functional natatory pleopods (Bernard 1953).
Despite the Eryoneicus name was suppressed by the International Commission on Zoological Nomenclature (1965) (see also Holthuis 1962), the different larvae are still named referring to the old nomenclature given that no study has conclusively proved the assignment of any Eryoneicus to the corresponding adult species. Indeed, no information on polychelid larvae hatching in captivity was available until Guerao and Abelló (1996) described a first larval stage of P. typhlops. The larvae hatched by Guerao and Abelló (1996) had not yet extruded or only partially extruded the natatory setae of the cephalothoracic appendages, so the description may not reflect the actual morphology of the larvae when hatching under natural conditions. The smallest Eryoneicus sampled from the wild so far, with a carapace length (CL) of 2 mm, was attributed to the third larval stage of E. connus by Bernard (1953). Selbie (1914) also caught a “juvenile” stage of Eryonicus sp. which corresponds to an advanced zoeal stage (TL = 7 mm; Plate IV) with undeveloped pleopods. Later stages of Eryoneicus larvae have well-developed pleopods and fit the definition of megalopa in this respect (Williamson 1969). The concepts “post-larva”, “decapodid” and “megalopa” have been interchangeably used in many decapod larval descriptions to refer to the transition phase between pelagic larvae and benthic phases (Gurney 1942; Kaestner 1970; Felder et al. 1985; Anger 2001). In the present study, the general name “decapodid” is used to denote the final larval phase preceding the moult to the first juvenile stage and characterised by the existence of functional pleopods and uropods with long plumose natatory setae (Kaestner 1970).
The aim of this study is to provide new evidence on the occurrence, distribution and morphology of larval stages of P. typhlops. Complete morphological descriptions are provided for three zoeal and one decapodid stages, while identification of the first larval stage of P. typhlops is confirmed through DNA analyses. In addition, a comparison of our plankton-collected specimens with previous descriptions of the larval stages of other Polychelidae is included. Finally, information on the ecology of these larvae, their abundances during different seasons, as well as their bathymetric distribution in the aphotic layers of the water column is reported.
Materials and methods
Sampling
Two multidisciplinary research surveys were conducted off the Balearic Islands (Western Mediterranean) during late autumn (29 November to 18 December 2009) and summer (11–30 July 2010). The main objective of the surveys was to determine the taxonomic composition, abundance, structure and vertical distribution of the mero-planktonic community at two stations located off the north-west and south of Mallorca (Balearic and Algerian subbasin, respectively). The sampling sites were located over 200 and 900 m depth (shelf break and middle slope, respectively) and present different environmental conditions (Pinot et al. 2002; López-Jurado et al. 2008). At the southern station of Mallorca, the upper slope is irregular, with numerous small canyons, while it is smooth in the northern station (Acosta et al. 2002). Two additional polychelid zoeal stages were captured between 300 and 400 m depth over the slope of Blanes canyon (NW Mediterranean) during 2004 under the “Observation, analysis and modelling of the Mediterranenan Sea” (OAMMS-04) survey, and were also used for photographic record.
A total of 218 depth-stratified meso-zooplankton samples, which were integrated in 34 hauls, were used to analyse decapod larvae composition. From these, 18 samples were collected using a multi-net (HYDRO-BIOS) in 2009 and 16 samples using a multiple opening–closing net and environmental sensing system (MOCNESS) in 2010 (Olivar et al. 2012). In order to determine the vertical distribution of decapod larvae, a series of oblique hauls were performed at four stations for 36 h at each site during the surveys in 2009 and 2010. Each oblique haul was performed down to 200 m depth on the shelf break and 500 m (in summer) or 850 m (in late autumn) on the middle slope, and seven or five depth strata were sampled depending on the season (summer and late autumn, respectively). The thickness of these strata changed with bathymetry and season (sampling protocols as in Torres et al. 2014). Supra-benthos samples were collected with a rectangular net rigged in a beam-trawl and used to catch mega-benthic fauna within 0.6 m above the bottom, with a cod-end mesh size of 500 µm in late autumn and 1,000 µm during summer. The catch speed was three knots, and the effective tow duration was 30 min. Supra-benthic samples were preserved in ethanol 96 % immediately after collection (sampling protocols as in Herrera et al. 2014). Once in the laboratory, decapod crustacean larvae were sorted and identified to species level and developmental stage whenever possible, using available descriptions and keys (Dos Santos and González-Gordillo 2004). Information on the stations where polychelid larvae were found is presented in Table 1. The zoeal stages and decapodid have been deposited at the Biological Collections of Reference of the Institut de Ciències del Mar (CSIC) in Barcelona under accession numbers ICMD000049-56.
In order to obtain reference DNA sequences for larval identification, several adult specimens for both P. typhlops and S. sculpta were sampled from Mediterranean deep-sea waters. P. typhlops was sampled from the region under the MEDITS2011 research cruise, and S. sculpta was sampled in July 2010 from the Catalano-Balearic basin, between Barcelona and Mallorca. DNA sequences for specimens from Atlantic waters were obtained from GenBank (Table 2).
DNA analyses
Total genomic DNA extraction from the first zoea specimen captured during supra-benthos sampling south of the Balearic Sea (Station: 39.067N–2.675E; Table 1) and the adult specimens from Mediterranean waters was performed using the Chelex-resin method (Palero et al. 2010). The standard universal primers for DNA barcoding (Folmer et al. 1994) were used for PCR amplification, given that they had been previously tested in Polycheles with positive results (see Palero et al. 2009). Amplifications were carried out with ~30 ng of genomic DNA in a reaction containing 1 U of Taq polymerase (Amersham), 1× buffer (Amersham), 0.2 mM of each primer and 0.12 mM dNTPs. The PCR thermal profile used was 94 °C for 4 min for initial denaturation, followed by 30 cycles of 94 °C for 30 s, 50 °C for 30 s, 72 °C for 30 s and a final extension at 72 °C for 4 min. Amplified PCR products were purified with QIAGEN-QIAquick PCR Purification Kit (QIAGEN Inc) prior to direct sequencing of the product. The sequences were obtained using the Big-Dye Ready-Reaction kit v3.1 (Applied Biosystems) on an ABI Prism 3770 automated sequencer from the Scientific and Technical Services of the Centre for Public Health Research (Valencia, Spain).
The DNA sequence alignment was conducted using the program MUSCLE v3.6 (Edgar 2004) with default parameters and then checked by eye. Before carrying out the likelihood-based analysis, model selection of nucleotide substitution was performed with MEGA5 (Tamura et al. 2011) according to BIC scores (Bayesian Information Criterion) and AICc value (Akaike Information Criterion, corrected). The aligned dataset was then used to estimate maximum likelihood (ML) phylogenies under the selected DNA substitution model using MEGA5 (Tamura et al. 2011). Bootstrap branch support values were calculated with 500 ML replicates. The aligned dataset was also used in MEGA5 (Tamura et al. 2011) to estimate Kimura 2-Parameter (K2P) distances among DNA sequences of the larval specimen and adults from different polychelid species.
Morphological descriptions
Dissection and measurements were taken with a Nikon SMZ800 stereo microscope equipped with an image analysing system (AnalySIS, SIS, Münster, Germany). An Olympus BH-2 microscope was used in the observation of the features of the appendages. The following measurements were taken: CL was measured as the distance from the frontal margin to the posterior margin of the carapace; carapace width (CW) as the greatest distance across the carapace; total length (TL) was measured as the distance from the frontal margin of the carapace to the posterior tip of the telson. The number of individuals examined per stage varied between 1 and 4.
For scanning electron microscopy (SEM), two first zoeal stages were sonicated for 2–3 min for removal of surface debris and dehydrated in a graded ethanol series (70, 90 and 100 %). After critical point drying, individuals were mounted on SEM stubs with self-adhesive carbon stickers and were coated in gold. Dried specimens were observed with a Hitachi H-4100 FE SEM.
The long plumose setae on the distal exopod of the maxillipeds and pereiopods are drawn truncated for clarity. Larval descriptions follow the basic malacostracan body pattern from anterior to posterior, and setal armature on appendages is described from proximal to distal subdivisions and from endopod to exopod (Clark et al. 1998; Haug et al. 2013). The setal terminology used was established by Ingle (1993).
Results
Phylogenetic analyses
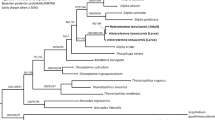
The new sequences for the Eryoneicus larva (Station: 39.067°N–2.675E; Table 1) and the adult samples used for molecular analyses have been deposited in GenBank with Accession numbers as shown in Table 2. The length of the aligned dataset for the COI gene was 679 bp and showed an excess for AT content (~60 %), as commonly found in mtDNA gene sequences. The TN93 + I DNA substitution model gave the lowest score under both the AICc (3,602.16) and the BIC (3,800.37), and therefore, it was used for subsequent ML searches. The phylogenetic tree obtained clearly showed the species-level assignment of the larvae, with the clade formed by the Eryoneicus specimen and the available P. typhlops adult specimens providing a 100 bootstrap support (Fig. 1). The K2P distance values observed when comparing the zoea collected from the plankton with either S. nana (21.7 %) or S. sculpta (24.8 %) fall within divergence levels observed among different genera, whereas the comparison with P. typhlops (0.17 %) is within the standard intra-specific distances observed in decapod crustaceans (see “Discussion”).
Morphological descriptions
The first zoeal stage and the decapodid stage are described in detail. For the zoeal stages II and III, only the main differences from the first zoea are presented.
Zoea I
Size: TL = 1.8–2.0 mm; CL = 1.4–1.6 mm; CW = 1.3–1.5 mm.
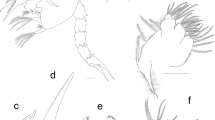
Carapace (Figs. 2a, b, 3a, b). Globose, almost spherical, much wider than the pleon, with numerous (55–60) ramified spines and long plumose setae. Two robust processes (column) are placed along the middorsal line, one is at middorsal carapace (C1, Fig. 2b, c) and the other is at the posterior part (C2, Fig. 2b, d, e). The arrangement of spines on middorsal line is R, 1, 1, 1, 2, C1, 2 C2 (see Fig. 3b). Frontal margin with a rostral spine (Fig. 3c, f), long and ramified (Fig. 2e). Vestigial eyes-stalk present. Details of the first spine of dorsal carina and antennal spine are shown in Fig. 2g, h.
Polycheles typhlops. First larval stage (ZI). a carapace, frontal view, indicating spines on middorsal line; b total animal, posterior view; c anterior column; d, e posterior column; f rostral spine; g first spine of dorsal carina; h antennal spine. r Rostral spine; c1 anterior column; c2 posterior column; t1 first pereiopod; t2 second pereiopod
Antennule (Fig. 3d). Not subdivided and conical, with two aesthetascs and three setae distally. Inner flagellum bud present.
Antenna (Fig. 3e). Biramous, not subdivided and without setae.
Mandible (Fig. 3f). Well-developed, showing no distinction between molar and incisor portions, with eight teeth. Not subdivided palp bud present.
Maxillule (Fig. 3g). Coxal endite with five plumo-denticulate setae (four terminal + one long setae in the inner margin). Basipodal endite with eight setae (three cuspidate + five plumo-denticulate).
Maxilla (Fig. 3h). A single lobe present with two simple setae. Exopod (scaphognathite) with 26–28 marginal plumose setae.
First maxilliped (Fig. 4a). Biramous. Protopod with 4 setae on the inner margin. Endopod not subdivided with four terminal plumose setae and one subterminal simple setae. Exopod not subdivided with four lateral and four long terminal plumose setae.
Second maxilliped (Fig. 4b). Biramous. Protopod with 6 setae in the inner margin. Endopod 3-subdivided with 2, 6, 5 setae. Exopod long (around three times longer than the endopod) with four long terminal plumose setae.
Third maxilliped (Fig. 4c). Biramous. Protopod with two setae. Endopod 5-subdivided with 1, 4, 4, 7, 5 setae. Exopod with six long terminal plumose setae.
First pereiopod (Fig. 4d). Biramous. Coxa with three setae. Basis with five setae. Endopod 4-subdivided and cheliform; ischio-merus (ischium and merus not separated) with four strong ramified spines and with a long plumose seta adjacent to each spine; carpus with two strong ramified spines and two distal simple small spines, and with a long plumose seta adjacent to each spine; propodus longer than the ischio-merus and with about 14 setae, including small setae on fixed finger; dactylus half the length of the propodus, apically curved with about ten setae randomly distributed on both margins. Exopod with six long plumose setae.
Second pereiopod (Fig. 4e). Biramous. The coxa was lost. Basis with four setae. Endopod four-subdivided and cheliform, shorter than the first pereiopod; ischio-merus with five strong ramified spines and with a long plumose seta adjacent to each spine; carpus half the length of the ischio-merus with two strong ramified spines and five simple minute spines, with a long plumose seta adjacent to each spine; propodus longer than carpus with several minute setae randomly distributed; dactylus 2/5 times the length of the propodus with several minute setae distributed as figured. Exopod with six long plumose setae.
Third pereiopod. Present as bud (not figured).
Fourth and fifth pereiopods: absent.
Pleon (Fig. 4f). Small and six-segmented. With a pair of postero-dorsal long sparsely setose setae on pleonites 3–6.
Minute pleopod buds are present on pleonites 2–6.
Telson: triangular, with two posterior minute processes on each side of the small concave posterior margin.
Zoea II
Size: TL = 3.3 mm; CL = 2.3 mm; CW = 2.2 mm.
Carapace (Figs. 5a, 6a, b). Frontal region with rostrum and a pairs of long (shorter than rostrum) ramified spines. The arrangement of spines on middorsal line is R, 1, 1, 1, 2, C1, 2, 2, C2, 2.
Antennule (Fig. 6c). Outer flagellum two-subdivided, with two subterminal aesthetascs and three terminal setae on distal subdivision. Inner flagellum longer than previous stage.
Antenna (Fig. 6d). Incipiently subdivided. Renal bud process present.
Mandible (Fig. 6e). Now with ten teeth.
Maxilla. Exopod (scaphognathite) with 33 marginal plumose setae.
Second maxilliped. Protopod with seven setae in the inner margin. Endopod three-subdivided with 3, 6, 5 setae.
Third maxilliped. Endopod five-subdivided with 2, 6, 4, 7, 5 setae.
Second pereiopod. Ischio-merus with one additional simple spine in the inner margin. Carpus with one additional minute distal spine.
Third pereiopod. Biramous, not subdivided and unarmed.
Pereiopods 4 and 5. Uniramous, present as a bud.
Pleon (Fig. 6f–h). Pleonites completely differentiated.
Pleopods (Fig. 6g) Biramous buds on pleonites 2–5. Pleonite six with biramous uropod buds.
Telson (Fig. 6h). Unarmed, two times longer than wide, posterior end 1/3 length of anterior part.
Zoea III
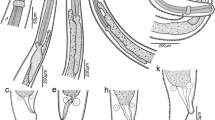
Size: TL = 4.7 mm; CL = 3.3 mm; CW = 3.4 mm.
Carapace (Figs. 5b–d, 7a, b). Cervical groove and branchial carinae incipiently developed. The number of the spines increases, many scattered between carinae.
Antennule (Fig. 7c). Biramous. Statolith present in the peduncle, with two short spines. Inner flagellum incipiently three-subdivided with 0, 2, 2 aesthetascs and 0, 0, 3 setae. Outer flagellum not subdivided with 3 terminal setae.
Antenna (Fig. 7d). Biramous. Exopod incipiently subdivided, without setae; endopod not subdivided, shorter than renal process and with one terminal setae.
Mandible (Fig. 7e). Now with ten teeth. Palp two-subdivided with a simple seta on distal subdivision.
Maxilla (Fig. 7f). Two endites with three and two setae, respectively. Scaphognathite with 50–54 plumose marginal setae (not figured).
First maxilliped. Protopod with five setae on the inner side. Exopod not subdivided with eight plumose setae.
Second maxilliped. Protopod with nine setae. Endopod three-subdivided with 6, 8, 5 setae.
Third maxilliped. Protopod with six setae. Endopod five-subdivided with 4, 12, 6, 10, 5 setae.
First pereiopod (Fig. 7g). Ischio-merus with four strong ramified spines; carpus with five spines (two strong ramified + three simple); propodus with seven simple spines. Setation as shown.
Second pereiopod (Fig. 7h). Ischio-merus with nine spines (five strong ramified + four simple); carpus with eight spines (two strong ramified + six simple). Setation as shown.
Pereiopods 3–5. Short and not subdivided.
Pleon (Fig. 7i–k). Small. First pleonite with one dorsal simple setae; second pleonite with a small postero-dorsal process and two long plumose setae; pleonites 3–5 each with one long postero-dorsal process, two long plumose setae and two simple setae; pleonite six with one long postero-dorsal process, two long and two small setae; all pleonites with rounded pleura, except the sixth that ends with a small process.
Pleopods (Fig. 7i, k). Without setae, propodus incipiently separated from the ramus; endopod presenting a small appendix interna.
Telson (Fig. 7i, j). Triangular shape in dorsal view, ending in a sharp median point, with one long antero-dorsal spine and two small simple setae on dorsal margin, lateral margins with 7–8 spines on each side.
Decapodid
Size: TL = 16.3 mm; CL = 8.5 mm; CW = 7.7 mm.
Carapace (Fig. 8a–c). Longer than wider, pear-shaped in dorsal view. Frontal margin with rostral spine simple, shorter than antennular peduncle. Orbital sinus well defined, internal angle of orbital sinus sparsely setose ending in a pointed process. Surface with more than 180 spines and long plumose setae, some in rows and carinae but many scattered between them. The arrangement of spines on median carina, between the rostral spine and the posterior margin is R, 1, 1, 1, 2, C1, 2, 2, C2, 2 (see Fig. 8a, b). Brachial region with 4 carinae; branchial upper carina with 11–13 spines; lateral carina with about 25 spines, including the antennal spine; longitudinal brachial carina with 6 spines in the posterior half of the carapace and about 24 minute spines in the anterior half of the carapace. Branchial lower carina with 17 small spines in the posterior half of the carapace; this carina does not reach the anterior part of the carapace. Eyestalks with a spine.
Antennule (Fig. 8d). Peduncle three-subdivided, basal subdivision flattened and enlarged with two distal long simple setae, the inner margin extends in the form of a long ridge triangular whose outer margin has about six long spines; posterior subdivisions unarmed. Outer flagellum approximately three times shorter than inner flagellum with 9–10 subdivisions, inner flagellum with 27 subdivisions.
Antenna (Fig. 8e). Renal process long, oblique, distally dilated. Scaphocerite short, lingulate, with 23–25 plumose setae. Flagellum of the endopod with 30 subdivisions.
Mandible (Fig. 8f). Similar to the zoeae with 14–15 triangular teeth, no show distinction between molar and incisor portions. Palp two-subdivided with 20–24 and more than 30 setae, respectively.
Maxillule (Fig. 8g). Coxal and basipodal endite with about 15 and 23 setae, respectively; without endopod.
Maxilla (Fig. 8h). Biramous, two maxillar lobes present, the smaller one with three distal simple setae and the longer one with 13 marginal simple setae. Scaphognathite large, with numerous marginal plumose setae.
First maxilliped (Fig. 9a). Endopod slender; exopodal lobe membraneous, reniform, extending further back than scaphognathite, exopod anteriorly divided into two lobes enclosing efferent passage.
Second maxilliped (Fig. 9b). Endopod four-subdivided densely setose.
Third maxilliped (Fig. 9c). Endopod five-subdivided densely setose, with vestigial epipod.
First pereiopod (Fig. 9d). First pereiopod very long, more robust than P2–5, ischium and merus now separated; spination as shown. Podobranch, epipod and two arthrobranch present.
Pereiopods 2–5 (Fig. 9e–h). Successively shorter posteriorly. Pereiopods 2–4 cheliform. Long spines present in ischio-merus and carpus of the second pereiopod (Fig. 9e). Third pereiopod with a distal long spine on carpus. Pereiopods 2–4 with podobranch, epipod, two arthrobranch and one pleurobranch present. Pereiopod five with one pleurobranch.
Pleon (Fig. 8a, b). Well-developed, spinulation indicated in Table 3; pleura of pleonites 1–2 rounded; pleura of pleonites 3–6 ending in a short sharp spine on the third and fourth but long and pointed on the fifth and sixth.
Pleopods (Fig. 9i). Biramous and functional; endopod with 30–32 plumose setae and bears an appendix interna with 10 coupling hooks; exopod with 34–36 plumose setae. Uropods functional, with numerous long plumose setae (endopod and exopod with more than 50 and 65, respectively).
Telson (Fig. 8a, b). Lanceolate in dorsal view, dorsal surface with one small and one strong spine placed anteriorly and several short simple setae randomly distributed. Each lateral margin with 6–9 spines.
Spatial and vertical distribution of Polycheles typhlops larvae
Twenty-five specimens of P. typhlops larvae were identified from samples taken in the Balearic Sea (Fig. 10). The larvae were captured mainly during the summer season (2010) but also in late autumn (2009). Relevant information about sampling details, such as location of sampling sites, density of larvae, date, time of sampling, water depth stratum and bottom depth is shown in Table 1. All zoea larvae and decapodid stages were found below the 200 m depth (Table 1; Fig. 11). Additionally, one first zoea stage was captured in the upper slope, near the bottom in the supra-benthos compartment (Table 1). Regarding their vertical distribution, the first zoeal stage could be found from 200 to 600 m depth, but mean abundances were higher in the layer between 300 and 500 m depth (Fig. 11) and in the southern study area. The last two zoeal stages were captured in a shallower layer (200–450 m depth), while the decapodid stage was collected near the bottom, between 600 and 800 m depth, in the north-west area. The vertical profiles of fluorescence during the late autumn survey were homogeneously distributed in the south and the north-west, ranging between 0.1 and 0.3 mg/m3 (Fig. 11a). Higher values were observed during the summer, with values ranging between 0.05 and 1.03 mg/m3 (Fig. 11b) and the presence of clines.
Study area with haul’s position during late autumn 2009 (open circle) and summer 2010 (open triangle), at four stations located over shelf break (250 isobath) and middle slope (900 isobath) off the north-west area and south of Mallorca Island. Grey lines indicate isobaths (200, 400, 600, 800 and 1,000 m)
Seasonal fluorescence (late autumn left; summer right) vertical profiles at north-west in black (Nslope_Fluo) and south middle slope in grey (Sslope_Fluo) from surface down to maximum sampled depth, adapted from Torres et al. (2014). Mean seasonal densities ontogenetic distributions of Polycheles typhlops larvae in the water column (depth mean) during the late autumn (black) and summer (grey) cruises, at north-west (N) and south (S) over middle slope (ZI first larval stage, ZII second larval stage, ZIII third larval stage, D decapodid)
Discussion
Morphology of Polycheles typhlops larvae
Accurate identification of marine larvae has traditionally required the rearing of larval stages in aquaria, but the development of species-specific markers (DNA barcoding) facilitates now the assignment of wild-caught planktonic larvae (Palero et al. 2008; Marco-Herrero et al. 2013). Matzen da Silva et al. (2011) have recently shown that the standard DNA barcoding COI gene region resolves relationships among decapod crustaceans. In their study, the observed mean K2P distance values did range from 0.29 to 1.38 % within species, 6.38–20.92 % within genus and 11.39–25.62 % within family. The K2P distance values found here when comparing our smallest zoea specimen (Station: 39.067 N–2.675E; Table 1) with either S. nana (21.7 %) or S. sculpta (24.8 %) fall within divergence levels observed among different genera, whereas the comparison with P. typhlops (0.17 %) is well within the K2P distance observed inside species. The molecular phylogeny also showed significant statistical support for the clustering of the larval sequence with DNA sequences obtained from adult specimens of P. typhlops. Therefore, the genetic results obtained in the present study, together with the fact that Stereomastis and Polycheles are the only polychelid genera known to occur in the Mediterranean, indicate that the first zoea larva collected from Western Mediterranean waters corresponds to P. typhlops. The larval development of P. typhlops is found to include at least three zoeal and one decapodid stages. Despite no molecular confirmation was made for the identity of ZII–ZIII and decapodid stages, species identification is inferred on morphological evidence (spination on the anterior part of middorsal line along the larval development and presence of epipodites on the decapodid stage). Following Ahyong (2009), the presence of epipodites on maxilliped three and pereiopods is used as one of the key features that allow for discrimination between the genera Stereomastis and Polycheles.
The smaller larvae of P. typhlops presented in this study were assigned to the first zoea (ZI) stage because they showed similar size (~1 mm CL) and the same degree of development as the first zoeal stage described from material reared in the laboratory (Guerao and Abelló 1996). The first zoea of P. typhlops had in both cases well-developed first and second pereiopods (biramous) and rudimentary pleopods. However, the description by Guerao and Abelló (1996) may not reflect the actual morphology of the larvae when hatching under natural conditions, given that many spines and setae on the carapace and appendages were not yet extruded. The degree of development indicates that the two later zoeae described here may correspond to the second (ZII) and third (ZIII) zoeal stages. These stages have well-developed interorbital spines, which are tiny in the first stage, pereiopods 4–5 present as buds and biramous pleopods. In the third zoeal stage, the pleopods are much more developed, even though the number of functional pereiopods does not increase. The main features that separate stages ZII and ZIII are the presence of appendix interna on the pleopods (but ramus without setae) in the third zoea, antennal exopod incipiently subdivided, telson triangular and setal development. From our observations, the zoea of Polychelidae are characterized by the presence of natatory exopods on the appendages of the pereion (maxillipeds and pereiopods), rostrum projecting and the absence of functional pleopods (see also Bernard 1953; Williamson 1983). The morphology and size of the most advanced zoea and the decapodid indicate that intermediate larval stages should exist between these two. In fact, the carapace of the decapodid is ~150 % longer than the zoea III carapace, while the increasing progression in size of the carapace among the zoeal stages does not exceed 44 %. The morphology of Eryoneicus larvae appears to change gradually, and no true metamorphosis has been observed between different stages (Bernard 1953; Williamson 1983). The most dramatic change that occurs between ZIII and the decapodid, besides the change in relative size of the pleon, is the appearance of well-developed and uniramous pereiopods.
Early stages of Eryoneicus species are seldom captured, and the complete zoeal development of a polychelid lobster is still unknown (e.g. Balss 1925; Stephensen 1935; Bernard 1953). The first description of a zoeal stage was reported by Selbie (1914) as a “juvenile” Eryonicus sp. from NW Atlantic waters. According to Selbie (1914): “This very interesting specimen was taken by the midwater otter trawl off the south-west coast; at the same station a small E. Faxoni was taken, and it is possible that the present specimen belongs to the same species”. Indeed, the zoea described by Selbie (1914) presented an arrangement of spines on the anterior part of the middorsal line (R, 1, 2, C1) clearly different from that found in our zoeal and decapodid stages (R, 1, 1, 1, 2, C1) and belongs probably to another species. Other early-stage Eryoneicus larvae described by Balss (1925) from Valdivia (SE Atlantic) and from the Arctic by Stephensen (1935) do not correspond to the zoeae of P. typhlops. Nevertheless, the decapodid described in the present study agrees very well with the description of E. puritanii given by Bouvier (1917). Only small differences were noted compared with Bouvier’s account (Table 3), such as the branchial lower carina not ending at the longitudinal carina, no pre-cervical grooves present, and minor differences in the spinulation pattern (see Table 3; Fig. 8a, b). In our decapodid stage, the dorsal spine of the first pleonite is forked at the base, and there are two median pleural spines in pleonites 3–5 and pleonite six bears 3 minute spines dorsally. These small differences could be attributed to the fact that the decapodid phase may include various stages, of which the latter would be neotenic forms with secondary sexual characteristics (see Williamson 1983).
Descriptions of E. puritanii specimens by Lo Bianco (1903), Bouvier (1917) and Bernard (1953) have been previously attributed to P. typhlops (Bouvier 1940; Kotthaus 1966). Lo Bianco (1903) samples were captured in the Gulf of Napoli (Western Mediterranean Sea), but several specimens attributed to E. puritanii have also been captured along the eastern Atlantic Ocean (Bernard 1953; Kotthaus 1966; Hernández and Tiefenbacher 1999; Hernández et al. 2007). E. puritanii larvae described by Bernard (1953) were ascribed to P. typhlops by Bouvier (1940) and Kotthaus (1966). Despite Bernard’s description (1953; Fig. 21) does not fit present standards, it seems to correspond to our second zoeal stage. Regarding the comparison of our decapodid with Lo Bianco’s original description, a different telson was figured (with a terminal spine) and therefore his description may not correspond to a decapodid stage of P. typhlops (Lo Bianco 1903, see Fig. 25 plate 8). Recall here that the decapodid (megalopa; see Anger 2001) denotes the final larval phase preceding moulting to the first juvenile stage and it is characterized by the existence of functional pleopods and uropods, subdivided and with long plumose natatory setae. Apart from E. puritanii catches by Lo Bianco (1903), other Eryoneicus forms were captured in the Mediterranean, namely the E. faxoni and E. kempi forms (Williamson 1983). The descriptions for E. kempi (Selbie 1914) and E. puritanii (Bouvier 1917) are similar, sharing the spine formula on the middorsal line (Bernard 1953). However, several differences can be observed between both species, such as the long spines and basal subdivision of the antennules or the cheliform 5th pereiopod.
Spatial and vertical distribution of Polycheles typhlops larvae
Although occurrences of adult polychelid lobsters on the epibenthos of the middle slope are common in the study area (Ramón et al. 2014), P. typhlops larvae were rare among all the collected material and were found exclusively in aphotic layers, corresponding to the lowest fluorescence values (Torres et al. 2014). The highest peak of P. typhlops larval abundances during the summer agrees with the highest frequency of ovigerous females in the Mediterranean (Follesa et al. 2007), and the bi-seasonal presence of larvae is in agreement with the fact that P. typhlops males are sexually active during the whole year (Cabiddu et al. 2008; Gastoni et al. 2010). The occurrence of P. typhlops larvae in deep plankton just above the adult populations is also in accordance with previous larval records. Bernard (1953) had already noted that all the Eryoneicus forms were captured below the euphotic zone and pointed towards the possibility of vertical ontogenetic migrations. The present study confirmed that P. typhlops larvae inhabit waters below the euphotic layer and that the decapodid stage is to be found in the deepest layers. This pattern further supports the idea that the larvae descend into deeper waters throughout their development, approaching the bottom at the end of the last larval stage in order to search for a suitable place to settle (Marta-Almeida et al. 2008; Shanks 2009). The lack of accounts for zoeal stages of P. typhlops in the previous literature is probably related to the low frequency of plankton sampling on deep waters, given that plankton studies usually focus on the photic layer. The larvae included in this study were captured at depths (between 200 and 800 m) where the photosynthetically active radiation (PAR) does not penetrate (e.g. Crise et al. 1998) and where local hydrographic currents are weaker than in shallower layers (Pinot et al. 1996; Amores et al. 2013). By staying within this depth range and through depth-keeping mechanisms (Shanks and Brink 2005), P. typhlops larvae might avoid the passive transportation suffered by other deep species spreading their larvae to the surface layers (Marta-Almeida et al. 2008).
The highest early-zoea larval densities were observed in the south slope during the summer season, coinciding with the maximum values of surface fluorescence and organic matter fluxes. Organic matter mean content of settling material, opal and CaCO3 fluxes to the necto-benthic communities estimated during the same oceanographic surveys show that the major inputs of marine organic matter (phytoplankton blooms) took place during summer in the south, being lithogenic fraction higher in the north area (Pasqual et al. submitted). On the north-west study area, where the shelf is narrower and the slope is quite pronounced, the currents over the shelf create mixed conditions (Torres et al. 2014). Laboratory studies on captured bathyal echinoids indicate that an increase in gonad size in response to food enhancement could increase spawning production (Eckelbarger and Watling 1995), and a similar response could also explain the highest P. typhlops larval abundance in the southern slope. Stomach contents for E. puritanii taken between 500 and 2,500 metres deep showed that they are able to feed on cnidaria, cyanophyceae, diatoms or coccolithophores (Bernard 1953) and support the classical view of deep-sea organisms being nourished by a “rain” of organic detritus coming from surface waters (Agassiz 1888). The capacity of decapod larvae to feed on microorganisms (Anger 2001) would be crucial in aphotic layers, where most C and N is sequestered in prokaryotes and bacterial biomass is dominant over phytoplankton biomass (Cho and Azam 1990; Lasternas et al. 2010). These facts give light in understanding the presence of polychelid Eryoneicus in dark oligotrophic waters where the larvae could take advantages of faecal pellets of herbivorous organisms covered with bacteria (Marshall 1954).
Conclusions
Detailed morphological examination, analysis of DNA sequences and comparison with previous studies provide evidence to support the assignment of the ancient species E. puritanii to the larval stages of P. typhlops. The larvae of P. typhlops are found to possess functional cheliform pereiopods and undeveloped eyes from the early zoeal stages. Besides the arrangement of spines on the anterior part of the middorsal line and the results from the DNA analysis on the ZI stage, the clear presence of an epipodite on maxilliped three and the pereiopods provides further support to the connection between E. puritanii, our decapodid specimen and P. typhlops. The results obtained in this study provide new information on the distribution and abundance of larval stages for one of the key groups of deep-sea fauna. The scarcity of conclusive data in the previous literature indicates the need for further descriptions in conjunction with the use of molecular techniques. An improvement of our knowledge about the larval ecology and recruitment of deep-sea species will be of utmost importance for the management of bathyal fauna.
References
Abelló P, Cartes JE (1992) Population characteristics of the deep-sea lobsters Polycheles typhlops and Stereomastis sculpta (Decapoda: Polychelidae) in a bathyal mud community of the Mediterranean Sea. Mar Biol 114(1):109–117
Abelló P, Macpherson E (1991) Distribution patterns and migration of Lithodes ferox (Filhol) (Anomura: Lithodidae) off Namibia. J Crust Biol 11:261–268
Abelló P, Valladares FJ (1988) Bathyal decapod crustaceans of the Catalan Sea (northwestern Mediterranean). Mésogée 58:97–105
Acosta J, Canals M, López-Martínez J, Muñoz A, Herranz P, Urgeles R, Palomo C, Casamor JL (2002) The balearic promontory geomorphology (Western Mediterranean): morphostructure and active processes. Geomorphology 49(3–4):177–204
Agassiz A (1888) Three cruises of the United States Coast and Geodetic Survey steamer Blake in the Gulf of Mexico, in the Caribbean Sea, and along the Atlantic coast of the United States from 1877 to 1880. Bull Museum Comp Zool Harvard 1:1–314
Ahyong S (2009) The Polychelidan lobsters: phylogeny and systematic (Polychelida: Polychelidae). In: Martin JW, Crandall KA, Felder DL (eds) Decapod crustacean phylogenetics, crustacean issues 18. CRC Press, New York, pp 369–396
Ahyong ST, Brown DE (2002) New species and new records of Polychelidae from Australia (Crustacea: Decapoda). Raffles Bull Zool 50(1):53–80
Ahyong ST, Chan TY (2004) Polychelid lobsters of Taiwan (Decapoda: Polychelidae). Raffles Bull Zool 52:171–182
Ahyong ST, Galil BS (2006) Polychelidae from the southern and western Pacific (Decapoda, Polychelida). Zoosystema 28(3):757
Amores A, Monserrat S, Marcos M (2013) Vertical structure and temporal evolution of an anticyclonic eddy in the Balearic Sea (Western Mediterranean). J Geophys Res: Ocean 118:2097–2106
Anger K (ed) (2001) The biology of decapod crustacean larvae. Crustacean issues, vol 14. A.A. Balkema, Lisse, Tokyo
Balss H (1925) Macrura der Deutschen Tiefsee-Expedition. 1. Palinura. Astacura und Thalassinidea. pp 195–196
Bate CS (1888) Report on the Crustacea Macrura dredged by HMS challenger during the years 1873–1876. Rep Voy Chall 24:1–942
Bernard FR (1953) Decapoda Eryonidae (Eryoneicus et Willemoesia). Dana-Rep 37:1–93
Bouvier EL (1905) Palinurides et Eryonides recueillis dans l’Atlantique oriental pendant les campagnes de l’Hirondelle et de la Princesse-Alice. Bull Mus Oceanogr Monaco 28:1–7
Bouvier EL (1917) Crustacés décapodes (macroures marcheurs) provenant des campagnes des yachts Hirondelle et Princesse-Alice (1885–1915). Résultats Campagnes scientifiques Prince Albert I de Monaco, 50:1–140, pls 1–11
Bouvier EL (1940) Décapodes marcheurs. Faune de France, 37:1–404, figs 1–222, pls 1–14
Cabiddu SM, Follesa C, Gastoni A, Porcu C, Cau A (2008) Gonad development of the deep-sea lobster Polycheles typhlops (Decapoda: Polychelidae) from the central Western Mediterranean. J Crustacean Biol 28(3):494–501
Cartes JE (1993) Day–night feeding by decapod crustaceans in a deep-water bottom community in the Western Mediterranean. J Mar Biol Assoc UK 73:795–811
Cartes JE (1998) Dynamics of the bathyal benthic boundary layer in the northwestern Mediterranean: depth and temporal variations in macrofaunal–megafaunal communities and their possible connections within deep-sea trophic webs. Prog Oceanogr 41:111–139
Cartes JE, Abelló P (1992) Comparative feeding habits of polychelid lobsters in the Western Mediterranean deep-sea communities. Mar Ecol Prog Ser 84:139–150
Cartes J, Sardà F, Abelló P (1993) Decapod crustaceans collected by deep-water trawls (between 1000 and 2200 m) in the Catalan area (north-western Mediterranean). Bios 1(1):206–221
Cartes JE, Huguet C, Parra S, Sánchez F (2007) Trophic relationships in deep-water decapods of Le Danois bank (Cantabrian Sea, NE Atlantic): trends related with depth and seasonal changes in food quality and availability. Deep Sea Res I 54:1091–1110
Chan TY (2010) Annotated checklist of the world’s marine lobsters (Crustacea: Decapoda: Astacidea, Glypheidea, Achelata, Polychelida). Raffles Bull Zool 23:153–181
Cho BC, Azam F (1990) Biogeochemical significance of bacterial biomass in the oceans euphotic zone. Mar Ecol Prog Ser 63:253–259
Clark PF, Calazans DK, Pohle GW (1998) Accuracy and standardization of brachyuran larval descriptions. Invertebr Reprod Dev 33:127–144
Company JB, Sardà F (1998) Metabolic rates and energy content of deep-sea benthic decapod crustaceans in the Western Mediterranean Sea. Deep Sea Res I 45:1861–1880
Company JB, Sardà F (2000) Growth parameters of deep-water decapod crustaceans in the northwestern Mediterranean Sea: a comparative approach. Mar Biol 136:79–90
Company JB, Sardà F, Puig P, Cartes JE, Palanques A (2003) Duration and timing of reproduction in decapod crustaceans of the NW Mediterranean continental margin: is there a general pattern? Mar Ecol Prog Ser 261:201–216
Company JB, Maiorano P, Tselepides A, Politou CY, Plaity W, Rotllant G, Sardà F (2004) Deep-sea decapod crustaceans in the western and central Mediterranean Sea: preliminary aspects of species distribution, biomass and population structure. Sci Mar 68(3):73–86
Crise A, Crispi G, Mauri E (1998) A seasonal three-dimensional study of the nitrogen cycle in the Mediterranean Sea: part I. Model implementation and numerical results. J Mar Syst 18(1):287–312
Dos Santos A, González-Gordillo JI (2004) Illustrated keys for the identification of the Pleocyemata (Crustacea: Decapoda) zoeal stages, from the coastal region of south-western Europe. J Mar Biol Assoc UK 84:205–227
Eckelbarger K, Watling L (1995) Role of phylogenetic constraints in determining reproductive patterns in deep-sea invertebrates. Invertebr Biol 114:256–269
Edgar RC (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32:1792–1797
Felder DL, Martin JW, Goy JW (1985) Patterns in early postlarval development of decapods. Crustacean issues. Larval Growth 2:163–225
Follesa MC, Cabiddu S, Gastoni A, Cau A (2007) On the reproductive biology of the deep-sea lobster, Polycheles typhlops (Decapoda, Palinura, Polychelidae), from the central-western Mediterranean. Crustaceana 80:839–846
Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R (1994) DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol Mar Biol Biotechnol 3:294–299
Fredj G, Laubier L (1985) The deep Mediterranean benthos. In: Moraitou-Apostolopoulou M, Kiortsis V (eds) Mediterranean marine ecosystems. Plenum Press, New York, pp 109–146
Galil BS (2000) Crustacea Decapoda: review of the genera and species of the family Polychelidae Wood-Mason, 1874. In: Crosnier A (ed) Résultats des campagnes MUSORSTOM, vol 21. Mem Mus Nat Hist Nat (Fr.) 184:285–387
Gastoni A, Follesa MC, Mulas A, Porcu C, Cau A (2010) Observations on Polycheles Sculptus SI Smith, 1880 (Decapoda, Palinura, Polychelidae) from Sardinian Waters (Central Western Mediterranean). Crustaceana 83(4):443–456
Guerao G, Abelló P (1996) Description of the first larval stage of Polycheles typhlops (Decapoda: Eryonidea: Polychelidae). J Nat Hist 30(8):1179–1184
Gurney R (1942) Larvae of decapod Crustacea. The Ray Society, London
Haug JT, Maas A, Haug C, Waloszek D (2013) Chapter 2: Evolution of crustacean appendages. In: Watling L, Thiel M (eds) The natural history of Crustacea. Vol. 1. Functional morphology and diversity. Oxford University Press, Oxford
Hernández F, Tiefenbacher L (1999) The presence of Eryoneicus puritanii in waters off the Canary Islands (Reptantia, Decapoda, Polychelidae). Bocagiana 195:1–5
Hernández F, De Vera A, Eugenia ML (2007) Eryoneicus puritanii Lo Bianco, 1903 en aguas de las islas de Cabo Verde (Decapoda, Reptantia, Polychelidae). Vieraea 35:51–56
Herrera A, Gómez M, Packard TT, Reglero P, Blanco E, Barberá-Cebrián C (2014) Potential respiration estimated by electron transport system activity in deep-sea suprabenthic crustaceans off Balearic Islands (Western Mediterranean). J Mar Syst. doi:10.1016/j.jmarsys.2014.02.015
Holthuis LB (1962) Stereomastis bate, 1888 (Crustacea, Decapoda), proposed validation under the plenary powers. ZN (S.) 1497. Bull Zool Nomencl 19(3):182–183
International Commission on Zoological Nomenclature (1965) Opinion 705. Bull Zool Nomencl 51:111–115
Ingle RW (1993) Hermit crabs of the Northeastern Atlantic Ocean and the Mediterranean Sea. Chapman and Hall, London, p 502
Kaestner A (1970) Invertebrate zoology, vol 3. Willey, New York
Kotthaus A (1966) Erstnachweis von Polycheles typhlops (Decapoda reptantia) für isländische Gewässer. Helgoland Mar Res XIII(4):348–353
Lasternas S, Agustí S, Duarte CM (2010) Bacteria and phytoplankton abundance and viability across the Mediterranean Sea. Aquat Microb Ecol 60:175–191. doi:10.3354/ame01421
Lo Bianco S (1903) Le pesche abissali eseguite da F.A. Krupp col yacht “Puritan” nelle adiacenze di Capri ed in altre localita del Mediterraneo. Mitteilungen aus der Zoologischen Station Neapel 16:109–279, pis 7–9
López-Jurado JL, Marcos M, Monserrat S (2008) Hydrographic conditions affecting two fishing grounds of Mallorca Island (Western Mediterranean): during the IDEA Project (2003–2004). J Mar Syst 71:303–315
Maiorano P, Pastore M, D’Onghia G, Latorre F (1998) Note on the population structure and reproduction of Polycheles typhlops (Decapoda: Polychelidae) on the upper slope of the Ionian Sea. J Nat Hist 32:1609–1618
Marco-Herrero E, Torres AP, Cuesta JA, Guerao G, Palero F, Abelló P (2013) The systematic position of Ergasticus (Decapoda, Brachyura) and allied genera, a molecular and morphological approach. Zool Scr 42(4):427–439
Marshall NB (1954) Aspects of deep sea biology. Hutchinson, London, p 380
Marta-Almeida M, Dubert J, Peliz A, dos Santos A, Queiroga H (2008) A modelling study of Norway lobster (Nephrops norvegicus) larval dispersal in southern Portugal: predictions of larval wastage and self-recruitment in the Algarve stock. Can J Fish Aquat Sci 65:2253–2268
Matzen da Silva J, Creer S, dos Santos A, Costa AC, Cunha MR et al (2011) Systematic and evolutionary insights derived from mtDNA COI barcode diversity in the decapoda (Crustacea: Malacostraca). PLoS ONE 6(5):e19449. doi:10.1371/journal.pone.0019449
Maynou F, Cartes JE (1998) Daily ration estimates and comparative study of food consumption in nine species of deep-water decapod crustaceans of the NW Mediterranean. Mar Ecol Prog Ser 171:221–231
Mullineaux LS, Wiebe PH, Baker ET (1995) Larvae of benthic invertebrates in hydrothermal vent plumes over Juan de Fuca ridge. Mar Biol 122:585–596
Olivar MP, Bernal A, Moli B, Peña M, Balbín R, Castellón A, Miquel J, Massutí E (2012) Vertical distribution, diversity and assemblages of mesopelagic fishes in the Western Mediterranean. Deep-Sea Res Part I 62:53–69
Palero F, Guerao G, Abelló P (2008) Morphology of the final stage phyllosoma larva of Scyllarus pygmaeus (Crustacea: Decapoda: Scyllaridae), identified by DNA analysis. J Plankton Res 30:483–488
Palero F, Crandall KA, Abelló P, Macpherson E, Pascual M (2009) Phylogenetic relationships between spiny, slipper and coral lobsters (Crustacea, Decapoda, Achelata). Mol Phylogenet Evol 50(1):152–162
Palero F, Hall S, Clark PF, Johnston D, Mackenzie-Dodds J, Thatje S (2010) DNA extraction from formalin-fixed tissue: new light from the deep sea. Sci Mar 74:465–470
Pasqual C, Calafat A, López-Fernández P, Monserrat S, Pusecddu A, Amores A, Flexas MM (2014) Organic carbon fluxes to the nekton-benthic communities of the Mallorca continental slope. Comparison within a Mediterranean context. J Mar Syst (submitted for publication)
Pinot JM, Tintoré J, Gomis D (1996) Multivariate analysis of the surface circulation in the Balearic Sea. Prog Oceanogr 36(4):343–376
Pinot JM, López-Jurado JL, Riera M (2002) The CANALES experiment (1996–1998). Interannual, seasonal and mesoscale variability of the circulation in the Balearic Channels. Prog Oceanogr 55:335–370
Ramón M, Abelló P, Ordinas F, Massutí E (2014) Deep epibenthic communities in two contrasting areas of the Balearic Islands (Western Mediterranean). J Mar Syst 132:54–65. doi:10.1016/j.jmarsys.2014.01.002
Selbie CM (1914) The Decapoda Reptantia of the coasts of Ireland. Part I. Palinura, Astacura and Anomura (except Paguridea). Fish Irel Sci Invest I:1–116, 15 pls
Shanks AL (2009) Pelagic larval duration and dispersal distance revisited. Biol Bull 216:373–385
Shanks AL, Brink L (2005) Upwelling, downwelling, and cross-shelf transport of bivalve larvae: test of a hypothesis. Mar Ecol Prog Ser 302:1–12
Somerton DA (1981) Contribution to the life history of the deep-sea king crab, Lithodes couesi, in the Gulf of Alaska. Fish Bull 79:259–269
Stephensen K (1935) The Godthaab expedition 1928. Crustacea Decapoda. Meddelelser om GrØnland udgivne af Kommissionen for Videnskabelige Undersogelser i GrØnland 80(1):1–94
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739
Torres AP, Dos Santos A, Alemany F, Massutí E (2013) Larval stages of crustacean species of interest for conservation and fishing exploitation in the Western Mediterranean. Sci Mar 77(1):159–160. doi:10.5989/scimar.05759.56D
Torres AP, Dos Santos A, Balbín R, Alemany F, Massutí E, Reglero P (2014) Decapod crustacean larval communities in the Western Mediterranean: seasonal composition, horizontal and vertical distribution patterns. J Mar Syst. doi:10.1016/j.jmarsys.2013.11.017
Wenner EL (1979) Some aspects of the biology of deep-sea lobsters of the family Polychelidae (Crustacea, Decapoda) from the western North Atlantic. Fish Bull 77:435–444
Wigley RL, Theroux RB, Murray HE (1975) Deep-sea red crab, Geryon quinquedens, survey off northeastern United States. Mar Fish Rev 37:1–21
Williamson DI (1969) Names of larvae in the Decapoda and Euphausiacea. Crustaceana 16:210–213
Williamson DI (1983) Decapoda, larvae, VIII. Nephropidea, Palinuridea, and Eryonidea. Fich Ident Zooplancton 167/168:1–8
Zariquiey-Alvarez R (1968) Crustáceos Decápodos Ibéricos. Investigaciones Pesqueras 32:1–510
Acknowledgments
The research was carried out within the framework of the IDEADOS (CTM5008-05589-C05-01) Project funded by the Plan Nacional I + D+i. The authors are very grateful to all the colleagues and crew members who participated in the IDEADOS surveys and three anonymous reviewers. A.P. Torres acknowledges pre-doctoral FPI Fellowship support from the regional government of the Balearic Islands, Conselleria d’Educacció, Cultura i Universitats, selected as part of an operational programme co-financed by the European Social Fund.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Communicated by H.-D. Franke.
Asvin P. Torres and Ferran Palero have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Torres, A.P., Palero, F., Dos Santos, A. et al. Larval stages of the deep-sea lobster Polycheles typhlops (Decapoda, Polychelida) identified by DNA analysis: morphology, systematic, distribution and ecology. Helgol Mar Res 68, 379–397 (2014). https://doi.org/10.1007/s10152-014-0397-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10152-014-0397-0