Abstract
Background
Application of the Milan criteria is an effective strategy to select patients with hepatocellular carcinoma (HCC) for liver transplantation, but HCC recurrence is still a major concern. The aim of this study was to determine whether interleukin 6 (IL6) polymorphisms and clinical variables are potential predictors for HCC recurrence and prognosis after transplantation.
Methods
A total of 110 consecutive patients with HCC undergoing liver transplantation were enrolled in the study. Six tag single nucleotide polymorphisms in IL6 were genotyped in both the donors and recipients. Demographic characteristics, HCC features, and IL6 polymorphisms were assessed against HCC recurrence.
Results
Pretransplant hepatitis B virus DNA (P = 0.014), pretransplant serum alpha-fetoprotein (P = 0.035), number of nodules (P = 0.011), diameter of main nodule (P = 0.001), macrovascular invasion (P = 0.001), microvascular invasion (P = 0.001), HCC exceeding the Milan criteria (P < 0.001), and donor rs2069852 AA genotype (P = 0.010) were associated with HCC recurrence. Recurrence-free survival rate and overall survival rate were significantly lower (P = 0.011 and P = 0.026, respectively) in patients whose donor had the rs2069852 AA genotype than in those whose donor had the AG and GG genotypes. Independent risk factors for recurrence-free survival and overall survival were microvascular invasion (P = 0.003; P = 0.002), HCC exceeding the Milan criteria (P < 0.001; P = 0.001), and donor rs2069852 AA genotype (P = 0.002; P = 0.010).
Conclusions
Our data suggest that donor IL6 rs2069852 polymorphisms may be a potential genetic marker for HCC recurrence after liver transplantation in the Han Chinese population.
Similar content being viewed by others
Introduction
Primary liver cancer is the fifth most common cancer worldwide and the second leading cause of cancer mortality [1]. Hepatocellular carcinoma (HCC) is the most common histological type of primary liver cancer, accounting for 85–90 % of these malignancies [1]. Liver transplantation is a well-regarded therapeutic option for patients with HCC as it negates concerns for missing undetectable liver tumors, corrects underlying cirrhosis, and reduces the probability of postoperative liver failure [2, 3]. However, the recurrence of HCC, as a serious complication, greatly affects the survival of patients with HCC undergoing liver transplantation [4, 5].
There is currently no fully satisfactory prognostic model for HCC recurrence following liver transplantation [4]. Application of the Milan criteria (single tumor ≤5 cm in diameter, or no more than 3 nodules with none exceeding 3 cm in diameter; no vascular invasion; no extra-hepatic spread) has been widely accepted as an effective strategy to select candidates with HCC for liver transplantation, and only 15–20 % of patients with HCC who meet the Milan criteria develop HCC recurrence [6, 7]. However, the dependence of the Milan criteria on pretransplant diagnostic imaging results—without any consideration of other factors, such as pathology and genetic variations—is a major limitation [7]. Consequently, there is an urgent need to search for and identify other risk factors with the potential to facilitate an accurate prediction of HCC recurrence [7, 8].
Interleukin 6 (IL6) is a pleiotropic cytokine that has critical functions in the regulation of the biological responses of several target cells, including hepatocytes [9, 10]. IL6 promotes liver regeneration and exerts hepatoprotective effects against Fas-mediated injury and toxic damage in the liver by activating the signal transducer and activator of transcription 3 (STAT3) and the mitogen-activated protein kinase (MAPK) signaling pathways [11]. However, IL6 has been shown to be associated with risk for HCC [12–14]. Therefore, we speculate that IL6 may be a potential risk factor associated with HCC recurrence after liver transplantation.
The aim of this study was to evaluate whether IL6 gene polymorphisms of donors and/or recipients contribute to HCC recurrence and prognosis after liver transplantation in a Han Chinese population. Other potential clinical risk factors for HCC recurrence were also investigated.
Patients and methods
Study population
A total of 110 consecutive patients with hepatitis B virus (HBV)-related HCC undergoing orthotopic liver transplantation between December 2006 and December 2013 at Shanghai Jiao Tong University Affiliated First People’s Hospital were enrolled in this study. Of these, 97 were men and 13 were women, with a mean age of 48.8 ± 8.3 years. Forty-three patients received local treatments prior to liver transplantation, including trans-hepatic arterial embolization or chemotherapy, radiofrequency ablation, and ethanol injection. Combination therapy of nucleoside analogues and low-dose intramuscular hepatitis B immunoglobulin was used to treat the HBV. Nucleoside analog therapy via the oral route was started 2 weeks before transplantation, and HBIG was administered intramuscularly on a fixed dosing schedule of 2000 IU of HBIG in the anhepatic phase, followed by 800 IU daily for the next 6 days, then 800 IU weekly for 3 weeks, and finally 800 IU monthly thereafter. HBV recurrence was defined as the persistence of the HBV surface antigen for 3 weeks, as well as its reappearance in serum after transplantation. All patients received immunosuppressive treatment consisting of tacrolimus and mycophenolate mofetil, which was continued in combination with steroids or basiliximab after liver transplantation. Patients were followed up with at least one visit monthly for the first 6 months post-transplantation, and then every 2 months for the second 6 months, every 6 months for the second year, then annually thereafter. HCC recurrence was defined as: (1) evidence of recurrence on ultrasonography, computed tomography, magnetic resonance, or plain film images + biopsy, and/or (2) autopsy. Suspicious lesions in the liver or lung were resected or biopsied. Observation of bone pain and progression of growth were routinely used to replace biopsies for bone lesions. If an increase in alpha-fetoprotein (AFP) level was observed prior to HCC recurrence, the time of recurrence was recorded as the date at which the AFP level began to rise [15]. All patients were followed up until 30 June 2015. The mean follow-up duration for all patients was 35.5 ± 21.9 (range 4–86) months.
Selection and genotyping of tag single nucleotide polymorphisms
The pairwise tagging algorithm in the HapMap website (http://hapmap.ncbi.nlm.nih.gov/) was used to select a set of tag single nucleotide polymorphisms (SNPs) by using the criteria of a linkage disequilibrium cutoff of r 2 = 0.8 and a minor allele frequency of >0.2 based on the HapMap database (Data Rel 27 PhaseII+III, Feb09, on NCBI B36 assembly, dbSNP b126). A total of six tag SNPs (rs6946864, rs4719711, rs2069852, rs6952003, rs1524098, rs17147230) captured common variants spanning an approximate 45-kb region ranging from 20 kb upstream to 20 kb downstream of the IL6 gene.
Genomic DNA was extracted from liver samples obtained from both donors and recipients using an AllPrep DNA/RNA Mini kit (Qiagen, Hilden, Germany). The SNaPshot sequencing method was used for genotyping. Multiplex PCR was performed and PCR products were purified. SNaPshot single-base extension reactions were then carried out, and subsequent products were purified and electrophoresed on an ABI3730XL system (Applied Biosystems, Foster City, CA). The data were analyzed using GeneMapper software version 4.1 (Applied Biosystems).
Statistical analysis
Variables were presented as frequency or mean ± standard deviation. Student’s t test was used for quantitative variables. Pearson’s Chi-squared test or Fisher’s exact test, where appropriate, was used for qualitative variables. The Kaplan–Meier (log-rank test) method was used for survival analysis. Cox regression analysis using a forward likelihood ratio method was performed to identify the risk factors for recurrence-free survival and overall survival. The survival curves were drawn by GraphPad Prism software version 5.01 (GraphPad Software Inc., San Diego, CA). Statistical analysis was performed using SPSS software version 17.0 (IBM Corp., Armonk, NY). The Hardy–Weinberg equilibrium was calculated using PLINK software (version 1.07; http://pngu.mgh.harvard.edu/purcell/plink/). All tests were two-sided, and a statistically significant difference was considered when the P value was <0.05.
Results
Clinical characteristics
The clinical characteristics of the patients according to recurrence and non-recurrence of HCC after transplantation are shown in Table 1. The overall incidence of HCC recurrence was 44.5 % (49/110). Of the 49 patients with HCC recurrence, intrahepatic recurrence was observed in 38 patients (77.6 %) and extrahepatic recurrence/metastasis occurred in 31 patients (63.3 %). The extrahepatic recurrence/metastasis was located in the lung (46.9 %, 23/49), bone (26.5 %, 13/49), brain (8.2 %, 4/49), and abdomen (4.1 %, 2/49), respectively. There were significant differences between the patients with recurrence and those with non-recurrence in terms of pretransplant HBV DNA (>5 log10 copies/ml; P = 0.014), pretransplant serum AFP level (>200 ng/ml; P = 0.035), number of nodules (≥2 nodules; P = 0.011), diameter of main nodule (>5 cm; P = 0.001), macrovascular invasion (P = 0.001), microvascular invasion (P = 0.001), and HCC exceeding the Milan criteria (assessed by radiological findings prior to transplantation; P < 0.001). However, no between-group differences were found for age (P = 0.454), sex (P = 0.472), HBV recurrence (P = 0.508), cirrhosis (P = 1.000), local treatment before transplantation (P = 0.263), tumor differentiation (P = 0.077), and immunosuppression therapy (P = 0.945).
IL6 gene polymorphisms
The allele and genotype frequencies of the six studied tag SNPs of the IL6 gene are shown in Table 2. There were no significant differences in allele frequencies between donors and recipients (P > 0.05). All six SNPs were in Hardy–Weinberg equilibrium (P > 0.05).
Associations between IL6 gene polymorphisms and HCC recurrence
Donor rs2069852 polymorphisms were found to be differentially distributed between recurrence patients and non-recurrence patients. The incidence of HCC recurrence was remarkably higher in patients with the donor rs2069852 AA genotype than in those with the donor rs2069852 AG and GG genotypes (P = 0.010; Table 3). The incidence of extrahepatic recurrence/metastasis in patients with the donor rs2069852 AA genotype was 39.6 % (19/48), which was higher than that in patients with the donor rs2069852 AG and GG genotypes (19.4 %, 12/62); the difference between the two groups was also statistically significant (P = 0.019). The distribution of the other five donor SNPs (rs6946864, rs4719711, rs6952003, rs1524098, rs17147230) between recurrence patients and non-recurrence patients was not significantly different (P > 0.05; Table 3). Recipient IL6 gene polymorphisms were not found to be significantly differentially distributed between patients with HCC recurrence and non-recurrence (P > 0.05; Table 4).
The patients who met the Milan criteria and those who exceeded the Milan criteria were separately investigated. In patients who exceeded the Milan criteria, the incidence of HCC recurrence was remarkably higher in patients with donor rs2069852 AA genotype than in those with donor rs2069852 AG and GG genotypes (Table 5, P = 0.007). In patients who met the Milan criteria, both donor and recipient IL6 gene polymorphisms did not show significant distributions between patients with HCC recurrence and non-recurrence (P > 0.05).
Associations between donor rs2069852 polymorphisms and survival rates
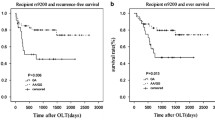
In patients with the donor rs2069852 AA genotype, the 1- and 5-year recurrence-free survival rates were 55.4 and 35.6 %, respectively, which were significantly lower than those of patients with the donor AG and GG genotypes (78.5 and 59.2 %, respectively; P = 0.011) (Fig. 1). The 1- and 5-year overall survival rates in patients with donor rs2069852 AA genotype were 75.0 and 40.6 %, respectively, compared to 83.9 and 61.6 %, respectively, in patients with the donor AG and GG genotypes. The difference between the two groups was statistically significant (P = 0.026) (Fig. 1).
In patients whose HCC exceeded the Milan criteria, the 1- and 5-year recurrence-free survival rates for those with the donor rs2069852 AA genotype were 36.8 and 8.5 %, respectively, compared to 66.0 and 45.0 %, respectively, for those with the donor AG and GG genotypes. The difference between the two groups was statistically significant (P = 0.010; Fig. 2). In patients whose HCC exceeded the Milan criteria, the 1- and 5-year overall survival rates for those with the donor rs2069852 AA genotype were 64.5 and 16.6 %, respectively, which were significantly lower than those with donor AG and GG genotypes (72.7 and 46.3 %, respectively; P = 0.039 (Fig. 2). In patients whose HCC met the Milan criteria, both the recurrence-free survival rate (P = 0.999) and the overall survival rate (P = 0.753) were not statistically different between patients with the donor rs2069852 AA genotype and those with the donor AG and GG genotypes.
Risk factors for survival rate by Cox regression analysis
The variables, including pretransplant HBV DNA (>5 log10 copies/ml), pretransplant serum AFP (>200 ng/ml), number of nodules (≥2 nodules), diameter of main nodule (>5 cm), macrovascular invasion, microvascular invasion, the Milan criteria, and donor rs2069852 polymorphisms, were subjected to Cox regression analysis. Microvascular invasion [hazards ratio (HR) 2.480, 95 % confidence interval (CI) 1.359–4.528; P = 0.003], HCC exceeding the Milan criteria (HR 4.506, 95 % CI 2.062–9.847; P < 0.001), and donor rs2069852 AA genotype (HR 2.513, 95 % CI 1.388–4.550; P = 0.002) were found to be independent risk factors for recurrence-free survival (Table 6). For overall survival, microvascular invasion (HR 2.729, 95 % CI 1.460–5.103; P = 0.002), HCC exceeding the Milan criteria (HR 4.015, 95 % CI 1.745–9.238; P = 0.001), and donor rs2069852 AA genotype (HR 2.239, 95 % CI 1.212–4.135; P = 0.010) were also independent risk factors (Table 7).
Discussion
Application of the Milan criteria in clinical practice has been shown to greatly reduce HCC recurrence rate to only 8 % and to improve the survival of HCC patients, with a 4-year recurrence-free survival rate of 83 % [16]. In our study, HCC meeting the Milan criteria was a protective factor for recurrence-free survival and overall survival. Our findings therefore support data from earlier studies which reported that application of the Milan criteria is an effective strategy to select patients with HCC for liver transplantation [16, 17]. However, it is well recognized that there is a substantial subset of HCC patients whose HCC exceeds the Milan criteria but who do have the potential for good outcomes after liver transplantation [18]. Several organ transplant centers have reported favorable outcomes when using expanded Milan criteria [19, 20]. On the other hand, although application of the Milan criteria effectively reduces HCC recurrence after liver transplantation, recurrence will remain a significant problem in coming years, despite its anticipated reduction to <20 % [7, 21]. To provide better predictive accuracy of HCC recurrence following liver transplantation, there is a need to identify new pathological and biological markers in addition to morphological tumor size and number [18].
In our study, pretransplant HBV DNA, pretransplant serum AFP, number of nodules, diameter of main nodule, and macrovascular invasion were associated with HCC recurrence, but they were not independent risk factors for survival. Li et al. found that HBV DNA levels before transplant are associated with HCC recurrence after transplantation [22], which is consistent with our findings; in contrast, a number of studies have found no association [23]. These discrepancies need to be explored. In Western countries and Japan, infection with hepatitis C virus (HCV) is the more common cause of HCC, while in China, HBV infection is the dominant risk factor [24], with Fan et al. reporting that about 90 % of HCC patients undergoing transplantation in Chinese transplant centers have HCC that is HBV-related [15]. Consequently, we study focused on HCC recurrence due to HBV infection. The correlation of IL6 gene polymorphisms with HCC recurrence due to HCV infection needs further validation. We found that number of nodules, diameter of the main nodule, and macrovascular invasion were significantly different between the patients with recurrence and those with non-recurrence; these clinical variables are in part common to the Milan criteria, and our findings thereby emphasize the significance of the Milan criteria once again. A prospective study showed that the predictive performance of the Milan criteria for HCC recurrence was significantly improved by a model that incorporated AFP level [25]. Rodriguez-Peralvarez et al. reported a slightly higher pretransplant serum AFP level in patients with HCC recurrence, but without significant difference [6]. Combined with our findings, pretransplant serum AFP level may to a certain extent be a useful biomarker to predict HCC recurrence following liver transplantation. The presence of microvascular invasion, as a marker of aggressive biological tumor behavior, affects the incidence of HCC recurrence after liver transplantation [26]. Our study demonstrates that microvascular invasion was a risk that increased HCC recurrence and affected the prognosis of HCC patients, which is consistent with previous findings [6, 26, 27].
A close relationship between cytokines and HCC has been reported in recent years [28–31]. IL6 is a crucial effector cytokine that is involved in hepatic physiology, including hepatoprotection, the acute-phase response, and mitogenesis in liver regeneration [11, 32]. This effect of IL6 is achieved through the STAT3 and MAPK signaling pathways by the binding of IL6 to its receptor complex (gp130-IL6R) on hepatocytes [11]. However, IL6 has been reported to be a molecular mediator for liver carcinogenesis by promoting hepatocyte proliferation and cell cycle progression [33]. A meta-analysis of eight case–control studies involving 1448 HCC cases and 3160 controls showed that IL6 gene polymorphisms are candidate risk factors for HCC susceptibility [13]. Ohishi et al. found that higher serum levels of IL6 were associated with an increased risk of HCC, independently of hepatitis virus infection, lifestyle-related factors, and radiation exposure [12]. In a mouse model, disruption of IL6 signaling was able to reduce risk of liver carcinogenesis [34]. Therefore, it is reasonable to speculate that IL6 plays an important role in the recurrence of HCC after liver transplantation.
Following liver transplantation, hepatocytes suffer from severe injuries that are in part caused by immunological responses. As an important component of the cytokine network, IL6 is involved in a complex series of events that control pathological and physiological changes after liver transplantation. IL6 gene polymorphisms have been shown to be predictive factors for acute rejection after liver transplantation [10, 35]. In our study population of Han Chinese patients with HCC, the donor IL6 rs2069852 AA genotype was found to be a predictor for an increased risk of HCC recurrence and a decreased survival rate, suggesting that IL6 has a tumor recurrence-promoting effect after liver transplantation. To our knowledge, our study is the first to describe the recurrence effect of the IL6 rs2069852 AA genotype. The functional behavior of rs2069852 has not yet been reported. It is known that the rs2069852 locus is in linkage disequilibrium with IL6 rs1800796 (r 2 = 0.83; HapMap datebase, Data Rel 27 PhaseII + III, Feb09, on NCBI B36 assembly, dbSNP b126) that is located in the IL6 gene promoter region, and a number of studies have demonstrated that rs1800796 is a functional SNP which regulates IL6 production [36–38]. We speculate that the IL6 rs2069852 AA genotype is associated with increased levels of IL6 which in turn lead to increased rates of HCC recurrence. However, the carcinogenesis effect of the IL6 rs2069852 AA genotype still needs validation.
In our study, donor IL6 rs2069852 polymorphisms, not the recipient counterparts, were found to be associated with HCC recurrence after liver transplantation. Wu et al. reported a lack of association between recipient cytokine gene polymorphisms, including IL1α, IL1β, IL6, IL8, IL10, tumor necrosis factor alpha and transforming growth factor beta-1, and HCC recurrence after liver transplantation in a Han Chinese patient population. One possible explanation for these results is that the authors did not evaluate the role of donor gene polymorphisms in HCC recurrence [39]. Norris et al. showed that hepatocytes could directly synthesize IL6 mRNAs and protein following hepatic stimuli, including partial hepatectomy [40]. Circulating tumor cells can lead to recurrence and metastasis of HCC after liver transplantation [41]. We speculate that donor hepatocytes alter not only local IL6 levels in the liver but also serum IL6 levels, both of which promote recurrence and metastasis after transplantation by acting on the circulating tumor cells.
In conclusion, microvascular invasion, HCC exceeding the Milan criteria, and the donor IL6 rs2069852 AA genotype may be effective predictive factors for HCC recurrence after liver transplantation in the Han Chinese population. These findings could help to tailor postoperative management strategies for patients with a high risk of HCC recurrence after transplantation.
References
Lafaro KJ, Demirjian AN, Pawlik TM (2015) Epidemiology of hepatocellular carcinoma. Surg Oncol Clin N Am 24:1–17
Zimmerman MA, Ghobrial RM, Tong MJ et al (2008) Recurrence of hepatocellular carcinoma following liver transplantation: a review of preoperative and postoperative prognostic indicators. Arch Surg 143:182–188
Fong ZV, Tanabe KK (2014) The clinical management of hepatocellular carcinoma in the United States, Europe, and Asia: a comprehensive and evidence-based comparison and review. Cancer 120:2824–2838
Bruix J, Gores GJ, Mazzaferro V (2014) Hepatocellular carcinoma: clinical frontiers and perspectives. Gut 63:844–855
Lai Q, Avolio AW, Lerut J et al (2012) Recurrence of hepatocellular cancer after liver transplantation: the role of primary resection and salvage transplantation in East and West. J Hepatol 57:974–979
Rodriguez-Peralvarez M, Tsochatzis E, Naveas MC et al (2013) Reduced exposure to calcineurin inhibitors early after liver transplantation prevents recurrence of hepatocellular carcinoma. J Hepatol 59:1193–1199
Welker MW, Bechstein WO, Zeuzem S et al (2013) Recurrent hepatocellular carcinoma after liver transplantation—an emerging clinical challenge. Transpl Int 26:109–118
Zheng Z, Gao S, Yang Z et al (2014) Single nucleotide polymorphisms in the metastasis-associated in colon cancer-1 gene predict the recurrence of hepatocellular carcinoma after transplantation. Int J Med Sci 11:142–150
Dickmann LJ, Patel SK, Rock DA et al (2011) Effects of interleukin-6 (IL-6) and an anti-IL-6 monoclonal antibody on drug-metabolizing enzymes in human hepatocyte culture. Drug Metab Dispos 39:1415–1422
Yao J, Feng XW, Yu XB et al (2013) Recipient IL-6-572C/G genotype is associated with reduced incidence of acute rejection following liver transplantation. J Int Med Res 41:356–364
Taub R (2004) Liver regeneration: from myth to mechanism. Nat Rev Mol Cell Biol 5:836–847
Ohishi W, Cologne JB, Fujiwara S et al (2014) Serum interleukin-6 associated with hepatocellular carcinoma risk: a nested case-control study. Int J Cancer 134:154–163
Liu Y, Gao SJ, Du BX et al (2014) Association of IL-6 polymorphisms with hepatocellular carcinoma risk: evidences from a meta-analysis. Tumour Biol 35:3551–3561
Falleti E, Fabris C, Toniutto P et al (2009) Interleukin-6 polymorphisms and gender: relationship with the occurrence of hepatocellular carcinoma in patients with end-stage liver disease. Oncology 77:304–313
Fan J, Yang GS, Fu ZR et al (2009) Liver transplantation outcomes in 1,078 hepatocellular carcinoma patients: a multi-center experience in Shanghai, China. J Cancer Res Clin Oncol 135:1403–1412
Mazzaferro V, Regalia E, Doci R et al (1996) Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 334:693–699
Lai Q, Lerut JP (2014) Hepatocellular cancer: how to expand safely inclusion criteria for liver transplantation. Curr Opin Organ Transplant 19:229–234
Takada Y, Tohyama T, Watanabe J (2015) Biological markers of hepatocellular carcinoma for use as selection criteria in liver transplantation. J Hepato Biliary Pancreat Sci 22:279–286
Yao FY, Ferrell L, Bass NM et al (2001) Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology 33:1394–1403
DuBay D, Sandroussi C, Sandhu L et al (2011) Liver transplantation for advanced hepatocellular carcinoma using poor tumor differentiation on biopsy as an exclusion criterion. Ann Surg 253:166–172
Clavien PA, Lesurtel M, Bossuyt PM et al (2012) Recommendations for liver transplantation for hepatocellular carcinoma: an international consensus conference report. Lancet Oncol 13:e11–e22
Li MR, Chen GH, Cai CJ et al (2011) High hepatitis B virus DNA level in serum before liver transplantation increases the risk of hepatocellular carcinoma recurrence. Digestion 84:134–141
Han SH, Reddy KR, Keeffe EB et al (2011) Clinical outcomes of liver transplantation for HBV-related hepatocellular carcinoma: data from the NIH HBV OLT study. Clin Transplant 25:E152–E162
Fitzmorris P, Shoreibah M, Anand BS et al (2015) Management of hepatocellular carcinoma. J Cancer Res Clin Oncol 141:861–876
Duvoux C, Roudot-Thoraval F, Decaens T et al (2012) Liver transplantation for hepatocellular carcinoma: a model including alpha-fetoprotein improves the performance of Milan criteria. Gastroenterology 143:986–994 (e983; quiz e914–985)
Iguchi T, Shirabe K, Aishima S et al (2015) New pathologic stratification of microvascular invasion in hepatocellular carcinoma: predicting prognosis after living-donor liver transplantation. Transplantation 99:1236–1242
Rodriguez-Peralvarez M, Luong TV, Andreana L et al (2013) A systematic review of microvascular invasion in hepatocellular carcinoma: diagnostic and prognostic variability. Ann Surg Oncol 20:325–339
Suo GJ, Zhao ZX (2013) Association of the interleukin-28B gene polymorphism with development of hepatitis virus-related hepatocellular carcinoma and liver cirrhosis: a meta-analysis. Genet Mol Res 12:3708–3717
Wei YG, Liu F, Li B et al (2011) Interleukin-10 gene polymorphisms and hepatocellular carcinoma susceptibility: a meta-analysis. World J Gastroenterol 17:3941–3947
Wei Y, Liu F, Li B et al (2011) Polymorphisms of tumor necrosis factor-alpha and hepatocellular carcinoma risk: a HuGE systematic review and meta-analysis. Dig Dis Sci 56:2227–2236
Li S, Deng Y, Chen ZP et al (2011) Genetic polymorphism of interleukin-16 influences susceptibility to HBV-related hepatocellular carcinoma in a Chinese population. Infect Genet Evol 11:2083–2088
Taki-Eldin A, Zhou L, Xie HY et al (2012) Liver regeneration after liver transplantation. Eur Surg Res 48:139–153
Johnson C, Han Y, Hughart N et al (2012) Interleukin-6 and its receptor, key players in hepatobiliary inflammation and cancer. Transl Gastrointest Cancer 1:58–70
Naugler WE, Sakurai T, Kim S et al (2007) Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science 317:121–124
Karimi MH, Daneshmandi S, Pourfathollah AA et al (2011) Association of IL-6 promoter and IFN-gamma gene polymorphisms with acute rejection of liver transplantation. Mol Biol Rep 38:4437–4443
Zhang G, Zhou B, Wang W et al (2012) A functional single-nucleotide polymorphism in the promoter of the gene encoding interleukin 6 is associated with susceptibility to tuberculosis. J Infect Dis 205:1697–1704
Komatsu Y, Tai H, Galicia JC et al (2005) Interleukin-6 (IL-6)–373 A9T11 allele is associated with reduced susceptibility to chronic periodontitis in Japanese subjects and decreased serum IL-6 level. Tissue Antigens 65:110–114
Brull DJ, Montgomery HE, Sanders J et al (2001) Interleukin-6 gene −174 g > c and −572 g > c promoter polymorphisms are strong predictors of plasma interleukin-6 levels after coronary artery bypass surgery. Arterioscler Thromb Vasc Biol 21:1458–1463
Wu LM, Zhou L, Xu J et al (2013) Lack of association between genetic polymorphisms in cytokine genes and tumor recurrence in patients with hepatocellular carcinoma undergoing transplantation. Hepatobiliary Pancreat Dis Int 12:54–59
Norris CA, He M, Kang LI et al (2014) Synthesis of IL-6 by hepatocytes is a normal response to common hepatic stimuli. PLoS One 9:e96053
Zhang Y, Shi ZL, Yang X et al (2014) Targeting of circulating hepatocellular carcinoma cells to prevent postoperative recurrence and metastasis. World J Gastroenterol 20:142–147
Acknowledgments
This work was supported by the Scientific Research Project of Science and Technology Commission of Shanghai Municipality (No. 134119a6300).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
No author has any conflict of interest.
Ethics statement
Written informed consent forms were obtained from all donors and recipients. The study was approved by the Ethics Committee of Shanghai Jiao Tong University and was conducted strictly under the guidelines of the Declaration of Helsinki.
Additional information
D. Chen and S. Liu are contributed equally to this work.
About this article
Cite this article
Chen, D., Liu, S., Chen, S. et al. Donor interleukin 6 gene polymorphisms predict the recurrence of hepatocellular carcinoma after liver transplantation. Int J Clin Oncol 21, 1111–1119 (2016). https://doi.org/10.1007/s10147-016-1001-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-016-1001-1