Abstract
Background
Combination chemotherapy with S-1 and irinotecan is one of the standard treatments for metastatic colorectal cancer (mCRC) in Japan. However, there are few alternative practical second-line therapies. We conducted a phase II trial to evaluate the efficacy and safety of the combination of S-1 and irinotecan plus bevacizumab as a second-line treatment for oxaliplatin-refractory mCRC.
Methods
Patients with mCRC who were previously treated with oxaliplatin-containing regimens were enrolled. Oral S-1 at a dose of 80 mg/m2 was administered twice daily for 2 weeks, followed by a 1-week drug-free interval. Irinotecan at a dose of 150 mg/m2 and bevacizumab at a dose of 7.5 mg/kg were administered on day 1. The primary endpoint was progression-free survival (PFS).
Results
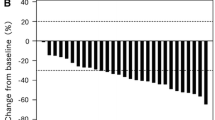
Thirty-seven patients were enrolled, and 34 and 36 patients were assessed for response and safety, respectively. The overall response rate was 20.6 % (95 % confidence interval [CI] 8.7–37.9), and the disease control rate was 76.5 % (95 % CI 58.8–89.3). The median PFS was 5.6 months (95 % CI 3.8–7.0). The median overall survival was 16.4 months (95 % CI 8.1–20.0). The most common grade 3/4 adverse events included neutropenia (25.0 %), anorexia (22.2 %), anemia (16.7 %), and fatigue/malaise (16.7 %). The most common grade 3/4 adverse event of special interest for bevacizumab was hypertension (30.6 %). One treatment-related death caused by gastrointestinal bleeding occurred.
Conclusions
The findings suggest that the combination of S-1 and irinotecan plus bevacizumab is effective and tolerable as second-line chemotherapy for patients with oxaliplatin-refractory mCRC.


Similar content being viewed by others
Change history
14 November 2017
In the original publication, in Abstract, the sentence that reads as, “Oral S-1 at a dose of 80 mg/m2 was…………. drug-free interval” should read as, “Oral S-1 at a dose of 40 mg/m2 was administered twice daily for 2 weeks, followed by a 1-week drug-free interval.
References
Shirasaka T, Shimamoto Y, Fukushima M (1993) Inhibition by oxonic acid of gastrointestinal toxicity of 5-fluorouracil without loss of its antitumor activity in rats. Cancer Res 53:4004–4009
Shirasaka T, Nakano K, Takechi T et al (1996) Antitumor activity of 1 M tegafur-0.4 M 5-chloro-2,4-dihydroxypyridine-1 M potassium oxonate (S-1) against human colon carcinoma orthotopically implanted into nude rats. Cancer Res 56:2602–2606
Shirasaka T, Shimamato Y, Ohshimo H et al (1996) Development of a novel form of an oral 5-fluorouracil derivative (S-1) directed to the potentiation of the tumor selective cytotoxicity of 5-fluorouracil by two biochemical modulators. Anticancer Drug 7:548–557
Miyamoto Y, Sakamoto Y, Yoshida N et al (2014) Efficacy of S-1 in colorectal cancer. Expert Opin Pharmacother 15:1761–1770
Goto A, Yamada Y, Yasui H et al (2006) Phase II study of combination therapy with S-1 and irinotecan in patients with advanced colorectal cancer. Ann Oncol 17:968–973
Yoshioka T, Kato S, Gamoh M et al (2009) Phase I/II study of sequential therapy with irinotecan and S-1 for metastatic colorectal cancer. Br J Cancer 101:1972–1977
Komatsu Y, Yuki S, Sogabe S et al (2012) Phase II study of combined chemotherapy with irinotecan and S-1 (IRIS) plus bevacizumab in patients with inoperable recurrent or advanced colorectal cancer. Acta Oncol 51:867–872
Yamada Y, Yamaguchi T, Matsumoto H et al (2012) Phase II study of oral S-1 with irinotecan and bevacizumab (SIRB) as first-line therapy for patients with metastatic colorectal cancer. Invest New Drug 30:1690–1696
Muro K, Boku N, Shimada Y et al (2010) Irinotecan plus S-1 (IRIS) versus fluorouracil and folinic acid plus irinotecan (FOLFIRI) as second-line chemotherapy for metastatic colorectal cancer: a randomised phase 2/3 non-inferiority study (FIRIS study). Lancet Oncol 11:853–860
Schmoll HJ, Van Cutsem E, Stein A et al (2012) ESMO Consensus Guidelines for management of patients with colon and rectal cancer. a personalized approach to clinical decision making. Ann Oncol 23:2479–2516
Wagner AD, Arnold D, Grothey AA et al (2009) Anti-angiogenic therapies for metastatic colorectal cancer. Cochrane Database Syst Rev. doi:10.1002/14651858
Strickler JH, Hurwitz HI (2014) Palliative treatment of metastatic colorectal cancer: what is the optimal approach? Curr Oncol Rep 16:363
Welch S, Spithoff K, Rumble RB et al (2010) Bevacizumab combined with chemotherapy for patients with advanced colorectal cancer: a systematic review. Ann Oncol 21:1152–1162
Baba H, Watanabe M, Okabe H et al (2012) Upregulation of ERCC1 and DPD expressions after oxaliplatin-based first-line chemotherapy for metastatic colorectal cancer. Br J Cancer 107:1950–1955
Komatsu Y, Yuki S, Sogabe S et al (2011) Phase II study of combined treatment with irinotecan and S-1 (IRIS) in patients with inoperable or recurrent advanced colorectal cancer (HGCSG0302). Oncology 80:70–75
Shiozawa M, Akaike M, Sugano N et al (2010) A phase II study of combination therapy with irinotecan and S-1 (IRIS) in patients with advanced colorectal cancer. Cancer Chemother Pharmacol 66:987–992
Oh SY, Ju YT, Choi SK et al (2011) Phase II study of irinotecan/S-1 combination chemotherapy for patients with oxaliplatin-refractory colorectal cancer. Invest New Drug 29:1050–1056
Saltz LB, Cox JV, Blanke C et al (2000) Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. N Engl J Med 343:905–914
Fuchs CS, Marshall J, Mitchell E et al (2007) Randomized, controlled trial of irinotecan plus infusional, bolus, or oral fluoropyrimidines in first-line treatment of metastatic colorectal cancer: results from the BICC-C Study. J Clin Oncol 25:4779–4786
Bennouna J, Sastre J, Arnold D et al (2013) Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): a randomised phase 3 trial. Lancet Oncol 14:29–37
Suenaga M, Nishina T, Mizunuma N et al (2015) Multicenter phase II study of FOLFIRI plus bevacizumab after discontinuation of oxaliplatin-based regimen for advanced or recurrent colorectal cancer (CR0802). BMC Cancer 15:176
Tsutsumi S, Ishibashi K, Uchida N et al (2012) Phase II trial of chemotherapy plus bevacizumab as second-line therapy for patients with metastatic colorectal cancer that progressed on bevacizumab with chemotherapy: the Gunma Clinical Oncology Group (GCOG) trial 001 SILK study. Oncology 83:151–157
Bendell JC, Tournigand C, Swieboda-Sadlej A et al (2013) Axitinib or bevacizumab plus FOLFIRI or modified FOLFOX-6 after failure of first-line therapy for metastatic colorectal cancer: a randomized phase II study. Clin Colorectal Cancer 12:239–247
Nakayama G, Uehara K, Ishigure K et al (2012) The efficacy and safety of bevacizumab beyond first progression in patients treated with first-line mFOLFOX6 followed by second-line FOLFIRI in advanced colorectal cancer: a multicenter, single-arm, phase II trial (CCOG-0801). Cancer Chemother Pharmacol 70:575–581
Iwamoto S, Takahashi T, Tamagawa H et al (2015) FOLFIRI plus bevacizumab as second-line therapy in patients with metastatic colorectal cancer after first-line bevacizumab plus oxaliplatin-based therapy: the randomized phase III EAGLE study. Ann Oncol 26:1427–1433
Suenaga M, Mizunuma N, Matsusaka S et al (2015) A phase I/II study of biweekly capecitabine and irinotecan plus bevacizumab as second-line chemotherapy in patients with metastatic colorectal cancer. Drug Des Devel Ther 9:1653–1662
Kubicka S, Greil R, André T et al (2014) Bevacizumab (BEV) plus chemotherapy (CT) continued beyond first disease progression (PD) in patients with metastatic colorectal cancer (mCRC) previously treated with BEV-based therapy: outcomes according to KRAS status and first-line CT backbone in the ML18147 study. J Clin Oncol 32(suppl 3):S520
Tabernero J, Yoshino T, Cohn AL et al (2015) Ramucirumab versus placebo in combination with second-line FOLFIRI in patients with metastatic colorectal carcinoma that progressed during or after first-line therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine (RAISE): a randomised, double-blind, multicentre, phase 3 study. Lancet Oncol 16:499–508
Van Cutsem E, Tabernero J, Lakomy R et al (2012) Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J Clin Oncol 30:3499–3506
Peeters M, Price TJ, Cervantes A et al (2010) Randomized phase III study of panitumumab with fluorouracil, leucovorin, and irinotecan (FOLFIRI) compared with FOLFIRI alone as second-line treatment in patients with metastatic colorectal cancer. J Clin Oncol 28:4706–4713
Acknowledgments
We thank all the patients and families who participated in this trial, and we are indebted to the physicians and all of the clinical study teams at the participating institutions. We also thank Ms. Sanae Sakamoto for her excellent secretarial assistance at the data center of Clinical Research Support Center Kyushu.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Y Miyamoto, H Tanioka, S Maekawa, H Kawanaka, M Kitazono, H Murakami, Y Ogata, H Saeki, M Shimokawa, S Natsugoe and Y Akagi have no conflict of interest. A Tsuji has received honoraria from Taiho Pharmaceutical. E Oki has received fees for promotional materials from Taiho Pharmaceutical, Yakult Honsha, Chugai Pharmaceutical and Merck Serono. Y Emi has received honoraria from Taiho Pharmaceutical, Chugai Pharmaceutical and Yakult Honsha. H Baba has received research funding and honoraria from Taiho Pharmaceutical, Chugai Pharmaceutical, Daiichi Sankyo. Y Maehara has received research funding and honoraria from Taiho Pharmaceutical, Chugai Pharmaceutical, Yakult Honsha and Daiichi Sankyo.
Additional information
A correction to this article is available online at https://doi.org/10.1007/s10147-017-1212-0.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10147_2015_943_MOESM1_ESM.ppt
Supplementary Fig. 1 Kaplan–Meier survival curve for progression-free survival (A) and overall survival (B) among patients receiving prior chemotherapy with or without bevacizumab. Bmab: bevacizumab (ppt 115 kb)
About this article
Cite this article
Miyamoto, Y., Tsuji, A., Tanioka, H. et al. S-1 and irinotecan plus bevacizumab as second-line chemotherapy for patients with oxaliplatin-refractory metastatic colorectal cancer: a multicenter phase II study in Japan (KSCC1102). Int J Clin Oncol 21, 705–712 (2016). https://doi.org/10.1007/s10147-015-0943-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-015-0943-z