Abstract
Background
Previous large trials with trastuzumab (TZM) showed improved outcome in patients with HER2-positive early-stage breast cancer. However, the efficacy and safety of TZM in Japanese patients have not been fully evaluated. We have therefore conducted an observational study in Japan.
Methods
This was a retrospective and a prospective observational study in which data on women with histologically confirmed HER2-positive invasive breast cancer who received TZM for stage I–IIIC disease were collected from 56 institutions that participated in the Japan Breast Cancer Research Group and the efficacy of each treatment regimen analyzed.
Results
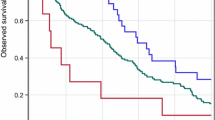
A total of 2,024 patients treated between July 2009 and June 2011 were initially enrolled in this study; in August 2013, the patient cohort comprised 2,009 patients. Of these, 142 (7.5 %) were aged ≥70 years, 1,097 (58.1 %) had clinically node-negative (cN0) breast cancer, and 883 (47.4 %) were estrogen receptor-positive. Treatment options were neoadjuvant therapy (662 patients) and adjuvant therapy with TZM (1,228 patients). Three-year overall survival (OS) rates in the entire cohort and in the neoadjuvant and adjuvant cohorts, respectively, were 98.9 [95 % confidence interval (CI) 98.2–99.3], 98.3 (95 % CI 96.8–99.1 %), and 99.2 % (95 % CI 98.4–99.6), respectively. Three-year disease-free survival (DFS) rates in the entire cohort and in the neoadjuvant and adjuvant cohorts, respectively were 94.2 (95 % CI 93.0–95.2), 94.8 (95 % CI 93.0–95.9), and 93.1 (95 % CI 90.7–94.9 %), respectively. Multivariate analysis showed that age and nodal status negatively correlated with DFS. Age was the only factor which correlated with OS rate. Adverse events (AEs) associated with TZM and grade 3/4 AEs were reported in 356 (18.8 %) and 14 (0.6 %) patients, respectively. Grade 3/4 cardiac toxicities were reported in 11 patients.
Conclusion
Based on data from our patient cohort of Japanese women with HER2-positive early-stage breast cancer, the efficacy and safety of systemic therapy with TZM are comparable to data from previously conducted large trials. Progress in anti-HER2 therapy for patients aged ≥70 years who have a poorer prognosis is needed.






Similar content being viewed by others
References
Gianni L, Dafni U, Gelber RD et al (2011) Treatment with trastuzumab for 1 year after adjuvant chemotherapy in patients with HER2-positive early breast cancer: a 4-year follow-up of a randomised controlled trial. Lancet Oncol 12:236–244
Slamon D, Eiermann W, Robert N et al (2011) Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med 365:1273–1283
Perez EA, Romond EH, Suman VJ et al (2011) Four-year follow-up of trastuzumab plus adjuvant chemotherapy for operable human epidermal growth factor receptor 2-positive breast cancer: joint analysis of data from NCCTG N9831 and NSABP B-31. J Clin Oncol 29:3366–3373
Joensuu H, Bono P, Kataja V et al (2009) Fluorouracil, epirubicin, and cyclophosphamide with either docetaxel or vinorelbine, with or without trastuzumab, as adjuvant treatments of breast cancer: final results of the FinHer Trial. J Clin Oncol 27:5685–5692
Spielmann M, Roche H, Delozier T et al (2009) Trastuzumab for patients with axillary-node-positive breast cancer: results of the FNCLCC-PACS 04 trial. J Clin Oncol 27:6129–6134
Jones SE, Collea R, Paul D et al (2013) Adjuvant docetaxel and cyclophosphamide plus trastuzumab in patients with HER2-amplified early stage breast cancer: a single-group, open-label, phase 2 study. Lancet Oncol 14:1121–1128
Goldhirsch A, Winer EP, Coates AS et al (2013) Personalizing the treatment of women with early breast cancer: highlights of the St Gallen international expert consensus on the primary therapy of early breast cancer 2013. Ann Oncol 24:2206–2223
Yamashiro H, Takada M, Nakatani E et al (2014) Prevalence and risk factors of bone metastasis and skeletal related events in patients with primary breast cancer in Japan. Int J Clin Oncol 19(5):852–862
Takada M, Ishiguro H, Nagai S et al (2014) Survival of HER2-positive primary breast cancer patients treated by neoadjuvant chemotherapy plus trastuzumab: a multicenter retrospective observational study (JBCRG-C03 study). Breast Cancer Res Treat 145:143–153
Geyer CE, Forster J, Lindquist D et al (2006) Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med 355:2733–2743
Swain SM, Kim SB, Cortes J et al (2013) Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA study): overall survival results from a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol 14:461–471
Verma S, Miles D, Gianni L et al (2012) Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med 367:1783–1791
Prat A, Cheang MC, Martin M et al (2013) Prognostic significance of progesterone receptor-positive tumor cells within immunohistochemically defined luminal A breast cancer. J Clin Oncol 31:203–209
Freedman RA, Vaz-Luis I, Barry WT et al (2014) Patterns of chemotherapy, toxicity, and short-term outcomes for older women receiving adjuvant trastuzumab-based therapy. Breast Cancer Res Treat 145(2):491–501
Sawaki M, Tokudome N, Mizuno T et al (2011) Evaluation of trastuzumab without chemotherapy as a post-operative adjuvant therapy in HER2-positive elderly breast cancer patients: randomized controlled trial [RESPECT (N-SAS BC07)]. Jpn J Clin Oncol 41:709–712
de Azambuja E, Procter MJ, van Veldhuisen DJ et al (2014) Trastuzumab-associated cardiac events at 8 years of median follow-up in the herceptin adjuvant trial (BIG 1-01). J Clin Oncol 32:2159–2165
Acknowledgments
The authors are grateful to all of the co-investigators and patients for their cooperation in the JBCRG C-01 study. They also thank the following additional investigators for their contributions to this study: K. Yamagami (Shinko Hospital), T. Morimoto (Yao Municipal Hospital), Y. Hasegawa (Hirosaki Municipal Hospital), H. Shigematsu (Hiroshima University Hospital), M. Hosoda (Hokkaido University Hospital), H. Abe (Bell Land General Hospital), D. Yotsumoto (Social Medical Corporation Hakuaikai Sagara Hospital), H. Tanino (Kitasato University Hospital), Y. Yamamoto (Kumamoto University Hospital), K. Hisamatsu (Oikawa Hospital), T. Aihara (Aihara Hospital), H. Bando (University of Tsukuba Hospital), H. Yoshibayashi (Japanese Red Cross Wakayama Medical Center), N. Tagaya (Dokkyo Medical University), H. Doihara (Okayama University Hospital), K. Narui (Yokohama City University Medical Center), H. Mukai (National Cancer Center Higashi Hospital), K. Aogi (National Hospital Organization Shikoku Cancer Center), S. Tsuyuki (Osaka Red Cross Hospital), Y. Kawabuchi (Hiroshima General Hospital), Y. Wada (National Hospital Organization Himeji Medical Center), Y. Kakugawa (Miyagi Cancer Center), Y. Moriguchi (Kyoto City Hospital), Y. Kawaguchi (Murakami Memorial Hospital Asahi University), H. Suwa (Hyogo Prefectural Tsukaguchi Hospital), F. Tanaka (Fukui Red Cross Hospital), H. Nakagomi (Yamanashi Prefectural Central Hospital), T. Ito (Rinku General Medical Center), S. Nakamura (Showa University Hospital), H. Takeuchi (Beppu Medical Center), M. Inokuchi (Kanazawa University Hospital), Y. Teramura (Hikone Municipal Hospital), K. Ito (Shinshu University School of Medicine), S. Sato (Ichinomiya Municipal Hospital), F. Yotsumoto (Shiga Medical Center for Adults), T. Okino (Kohka Public Hospital), Y. Mitsudo (Mitsubishi Kyoto Hospital), K. Yoshidome (Osaka Police Hospital), and Y. Tokunaga (Osakakita Teishin Hospital). The authors also thank Tetsuo Takeuchi for statistical work, Sachiko Inoue and Miyoko Hasebe for data management, and Kiyomi Kashiwa and Nobuko Aoki for secretarial work.
Conflict of interest
This study was conducted using our own funds from JBCRG. The Department of EBM Research, Institute for Advancement of Clinical and Translational Science was partially funded from Chugai Pharmaceutical Co., Ltd. H. Yamshiro and S. Ohno received an honorarium from Chugai, Novartis, and AstraZeneca. H. Iwata and R. Nishimura, N. Sato, T. Takano, S. Morita and M. Toi received an honorarium from from Chugai. N. Masuda received an honorarium from Chugai, Eisai and AstraZeneca. M. Takada received an honorarium from Eisai. H. Iwata, T. Takano, S. Yasuno, S. Morita and M. Toi received research funding from Chugai. N. Yamamoto received research funding from Pfizer Japan and Bayer. S. Yasuno received research funding from Pfizer Japan. H. Iwata recieved research funding from GSK. All other authors declare no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Yamshiro, H., Iwata, H., Masuda, N. et al. Outcomes of trastuzumab therapy in HER2-positive early breast cancer patients. Int J Clin Oncol 20, 709–722 (2015). https://doi.org/10.1007/s10147-015-0785-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-015-0785-8