Abstract
Background
Vacuolar-ATPases (V-ATPases) play an important role in maintaining a relatively neutral pHi (internal pH) and are responsible for the progression of cancer. V-ATPases contain different subunits and few studies have been conducted on subunit V1A. This study aimed to investigate the gene expression of V1A subunit of V-ATPases in gastric cancer tissues and explore its role in the progression and prognosis of gastric cancer.
Methods
The protein expressions of the V1A subunit of V-ATPase gene in 100 normal gastric specimens and 100 gastric cancer tissues were determined by immunohistochemistry. The role of V1A subunit of V-ATPases was studied using a specific small interfering RNA (siRNA).
Results
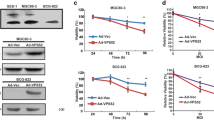
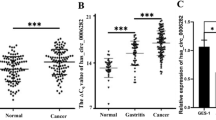
The positive expression rate of the V1A subunit of V-ATPases was 76 % in gastric cancer tissue samples, much higher than that in normal tissue samples (30 %, P < 0.05), and was correlated with histological grade (P = 0.001), lymph node metastasis (P = 0.002), TNM (P = 0.040), and vascular invasion (P = 0.010), but not with patient age, sex, depth of tumor invasion, tumor size, or histological type. The median overall survival times of 76 patients who had positive staining for tumor cell V1A subunit of V-ATPases and 24 patients who had negative staining were 31.7 and 59.2 months, respectively. When the expression of V1A subunit was knocked down using siRNA, the proliferation and invasion of gastric cancer cells in vitro were significantly inhibited.
Conclusions
V1A subunit of V-ATPases can be a prognostic indicator for poor outcome and is a therapeutic target in gastric cancer.







Similar content being viewed by others
References
Parkin DM, Bray F, Ferlay J et al (2005) Global cancer statistics, 2002. CA Cancer J Clin 55:74–108
Ushijima T, Sasako M (2004) Focus on gastric cancer. Cancer Cell 5:121–125
Kapadia CR (2003) Gastric atrophy, metaplasia, and dysplasia: a clinical perspective. J Clin Gastroenterol 36:S29–S36
Hinton A, Bond S, Forgac M (2009) V-ATPase functions in normal and disease processes. Pflugers Arch—Eur J Physiol 457:589–598
Perez-Sayans M, Suarez-Penaranda JM, Barros-Angueira F et al (2012) An update in the structure, function, and regulation of V-ATPases: the role of the C subunit. Braz J Biol 72:189–198
Petzoldt AG, Gleixner EM, Fumagalli A et al (2013) Elevated expression of the V-ATPase C subunit triggers JNK-dependent cell invasion and overgrowth in a Drosophila epithelium. Dis Model Mech 6:689–700
Sermoune SR, Bakunis K (2004) Vacuolar H+-ATPase in human breast human cancer cells with distinct metastatic potential: distribution and functional activity. Am J Physiol Cell Physiol 286:C1443–C1452
Sasazawa Y, Futamura Y, Tashiro E et al (2009) Vacuolar H+-ATPase inhibitors overcome Bcl-xL mediated chemoresistance through restoration of a caspase-independent apoptotic pathway. Cancer Sci 100:1460–1467
De Milito A, Fais S (2005) Tumor acidity, chemoresistance and proton pump inhibitors. Future Oncol 1:779–786
Hurtado-Lorenzo A, Skinner M, El Annan J et al (2006) V-ATPase interacts with ARNO and Arf6 in early endosomes and regulates the protein degradative pathway. Nat Cell Biol 8:124–136
Lu X, Qin W, Li J et al (2005) The growth and metastasis of human hepatocellular carcinoma xenografts are inhibited by small interfering RNA targeting to the subunit ATP6L of proton pump. Cancer Res 65:6843–6849
Hinton A, Bond S, Forgac M (2009) V-ATPase functions in normal and disease processes. Pflugers Arch 457:589–598
Clouston AD (2001) Timely topic: premalignant lesions associated with adenocarcinoma of the upper gastrointestinal tract. Pathology 33:271–277
Lim JH, Park JW, Kim MS et al (2006) Bafilomycin induces the p21-mediated growth inhibition of cancer cells under hypoxic conditions by expressing hypoxia-inducible factor-1alpha. Mol Pharmacol 70:856–865
Hong J, Yokomakura A, Nakano Y et al (2006) Inhibition of vacuolar-type (H+)-ATPase by the cytostatic macrolide apicularen A and its role in apicularen A-induced apoptosis in RAW 264.7 cells. FEBS Lett 580:2723–2730
Wojtkowiak JW, Rothberg JM, Kumar V et al (2012) Chronic autophagy is a cellular adaptation to tumor acidic pH microenvironments. Cancer Res 72:3938–3947
Huss M, Sasse F, Kunze B et al (2005) Archazolid and apicularen: novel specific V1A subunit of V-ATPases inhibitors. BMC Biochem 6:1–13
Pérez-Sayáns M, Somoza-Mart JM, Barros-Angueira F et al (2009) V-ATPases inhibitors and implication in cancer treatment. Cancer Treat Rev 35:707–713
Chen M, Zou X, Luo H et al (2009) Effects and mechanisms of proton pump inhibitors as a novel chemosensitizer on human gastric adenocarcinoma (SGC7901) cells. Cell Biol Int 33:1008–1019
De Milito A, Iessi E, Logozzi M et al (2007) Proton pump inhibitors induce apoptosis of human B-cell tumors through a caspase independent mechanism involving reactive oxygen species. Cancer Res 67:5408–5417
Dooley CM, Schwarz H, Mueller KP et al (2013) Slc45a2 and V-ATPase are regulators of melanosomal pH homeostasis in zebrafish, providing a mechanism for human pigment evolution and disease. Pigment Cell Melanoma Res 26:205–217
Sennoune SR, Luo D, Martinez-Zaguilan R (2004) Plasmalemmal vacuolar-type H(+)-ATPase in cancer biology. Cell Biochem Biophys 40:185–206
Tabares L, Betz B (2010) Multiple functions of the vesicular proton pump in nerve terminals. Neuron 68:1020–1022
Beyenbach KW, Wieczorek H (2006) The V-type H+ ATPase: molecular structure and function, physiological roles and regulation. J Exp Biol 2009:577–589
Garcia-Garcia A, Perez-Sayans Garcia M, Rodriguez MJ et al (2012) Immunohistochemical localization of C1 subunit of V-ATPase (ATPase C1) in oral squamous cell cancer and normal oral mucosa. Biotech Histochem 87:133–139
Acknowledgments
This work was supported by National Science Foundation Grant No. 81071816. Special thanks to Yong Liu and Junhao Chen for their technical assistance. We also thank Xingyun Xu for collecting materials and references.
Conflict of Interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Liu, P., Chen, H., Han, L. et al. Expression and role of V1A subunit of V-ATPases in gastric cancer cells. Int J Clin Oncol 20, 725–735 (2015). https://doi.org/10.1007/s10147-015-0782-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-015-0782-y