Abstract
Background
Deleted in breast cancer 1 (DBC1) was initially cloned from a region homozygously deleted in breast cancers, but its role in colorectal cancer remains unknown. The present study aims to examine the expression level of DBC1 and assess its prognostic value in human colorectal cancer.
Methods
Immunohistochemical staining was performed to detect the expression level of DBC1 in a series of 186 colorectal cancer patients. Immunohistochemical staining results were analyzed and compared statistically with various clinicopathological characters and overall survival.
Results
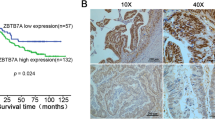
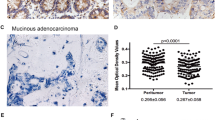
Compared with the corresponding non-tumor tissues, a higher expression level of DBC1 was detected in colorectal cancer (P < 0.01). Tissue microarray analysis revealed that DBC1 expression is significantly associated with tumor histological grade, TNM stage and metastatic status (P < 0.01). Importantly, Kaplan–Meier analysis showed that DBC1 expression is associated with shorter overall survival (P < 0.01). Univariate Cox regression suggested that DBC1 expression, poorly differentiation status and the presence of lymph node metastasis predict shorter overall survival in colorectal cancer (P < 0.05). Multivariate Cox regression analysis indicated that DBC1 acts as an independent prognostic factor in colorectal cancer (P < 0.01).
Conclusions
These results suggest that DBC1 is over-expressed in colorectal cancer and that it might serve as a predictor for selecting patients at high risk of poor prognosis.


Similar content being viewed by others
References
Kamangar F, Dores GM, Anderson WF (2006) Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol 24:2137–2150
Migliore L, Migheli F, Spisni R et al (2011) Genetics, cytogenetics, and epigenetics of colorectal cancer. J Biomed Biotechnol 2011:792362
Pritchard CC, Grady WM (2011) Colorectal cancer molecular biology moves into clinical practice. Gut 60:116–129
Pino MS, Chung DC (2011) Microsatellite instability in the management of colorectal cancer. Expert Rev Gastroenterol Hepatol 5:385–399
Chu D, Li Y, Wang W et al (2010) High level of Notch1 protein is associated with poor overall survival in colorectal cancer. Ann Surg Oncol 17:1337–1342
Hamaguchi M, Meth JL, von Klitzing C et al (2002) DBC2, a candidate for a tumor suppressor gene involved in breast cancer. Proc Natl Acad Sci USA 99:13647–13652
Lee H, Kim KR, Noh SJ et al (2011) Expression of DBC1 and SIRT1 is associated with poor prognosis for breast carcinoma. Hum Pathol 42:204–213
Sung JY, Kim R, Kim JE et al (2010) Balance between SIRT1 and DBC1 expression is lost in breast cancer. Cancer Sci 101:1738–1744
Cha EJ, Noh SJ, Kwon KS et al (2009) Expression of DBC1 and SIRT1 is associated with poor prognosis of gastric carcinoma. Clin Cancer Res 15:4453–4459
Kim SH, Kim JH, Yu EJ et al (2012) The overexpression of DBC1 in esophageal squamous cell carcinoma correlates with poor prognosis. Histol Histopathol 27:49–58
Kim JE, Sung S (2010) Deleted in breast cancer 1 (DBC1) is a dynamically regulated protein. Neoplasma 57:365–368
Sundararajan R, Chen G, Mukherjee C et al (2005) Caspase-dependent processing activates the proapoptotic activity of deleted in breast cancer-1 during tumor necrosis factor-alpha-mediated death signaling. Oncogene 24:4908–4920
Kim JE, Chen J, Lou Z (2009) p30 DBC is a potential regulator of tumorigenesis. Cell Cycle 8:2932–2935
Kim JE, Chen J, Lou Z (2008) DBC1 is a negative regulator of SIRT1. Nature 451:583–586
Trauernicht AM, Kim SJ, Kim NH et al (2010) DBC-1 mediates endocrine resistant breast cancer cell survival. Cell Cycle 9:1218–1219
Trauernicht AM, Kim SJ, Kim NH et al (2007) Modulation of estrogen receptor alpha protein level and survival function by DBC-1. Mol Endocrinol 21:1526–1536
Suen TC, Goss PE (2001) Identification of a novel transcriptional repressor element located in the first intron of the human BRCA1 gene. Oncogene 20:440–450
Hiraike H, Wada-Hiraike O, Nakagawa S et al (2010) Identification of DBC1 as a transcriptional repressor for BRCA1. Br J Cancer 102:1061–1067
Garapaty S, Xu CF, Trojer P et al (2009) Identification and characterization of a novel nuclear protein complex involved in nuclear hormone receptor-mediated gene regulation. J Biol Chem 284:7542–7552
Fu J, Jiang J, Li J et al (2009) Deleted in breast cancer 1, a novel androgen receptor (AR) coactivator that promotes AR DNA-binding activity. J Biol Chem 284:6832–6840
Gomez I, Pena C, Herrera M et al (2011) TWIST1 is expressed in colorectal carcinomas and predicts patient survival. PLoS ONE 6:e18023
Chu D, Zhang Z, Li Y et al (2011) Prediction of colorectal cancer relapse and prognosis by tissue mRNA levels of NDRG2. Mol Cancer Ther 10:47–56
Ogino S, Shima K, Baba Y et al (2009) Colorectal cancer expression of peroxisome proliferator-activated receptor gamma (PPARG, PPARgamma) is associated with good prognosis. Gastroenterology 136:1242–1250
Grady WM, Carethers JM (2008) Genomic and epigenetic instability in colorectal cancer pathogenesis. Gastroenterology 135:1079–1099
Popat S, Hubner R, Houlston RS (2005) Systematic review of microsatellite instability and colorectal cancer prognosis. J Clin Oncol 23:609–618
Nosho K, Kure S, Irahara N et al (2009) A prospective cohort study shows unique epigenetic, genetic, and prognostic features of synchronous colorectal cancers. Gastroenterology 137(1609–1620):e1601–e1603
Zhao W, Kruse JP, Tang Y et al (2008) Negative regulation of the deacetylase SIRT1 by DBC1. Nature 451:587–590
Brooks CL, Gu W (2009) How does SIRT1 affect metabolism, senescence and cancer? Nat Rev Cancer 9:123–128
Venkitaraman AR (2009) Linking the cellular functions of BRCA genes to cancer pathogenesis and treatment. Annu Rev Pathol 4:461–487
Soussi T (2000) p53 Antibodies in the sera of patients with various types of cancer: a review. Cancer Res 60:1777–1788
Reid JF, Gariboldi M, Sokolova V et al (2009) Integrative approach for prioritizing cancer genes in sporadic colon cancer. Genes Chromosomes Cancer 48:953–962
Macartney-Coxson DP, Hood KA, Shi HJ et al (2008) Metastatic susceptibility locus, an 8p hot-spot for tumour progression disrupted in colorectal liver metastases: 13 candidate genes examined at the DNA, mRNA and protein level. BMC Cancer 8:187
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Nos. 81090270, 81090273, 81000864 and 81101891) and the National Municipal Science and Technology Project (2009ZX09103-667 and 2009ZX09301-009-RC06).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Y. Zhang and Y. Gu contributed equally to this work.
About this article
Cite this article
Zhang, Y., Gu, Y., Sha, S. et al. DBC1 is over-expressed and associated with poor prognosis in colorectal cancer. Int J Clin Oncol 19, 106–112 (2014). https://doi.org/10.1007/s10147-012-0506-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-012-0506-5