Abstract
Bat hibernacula with high numbers of bats can become high-risk areas, as they attract flying and non-flying predators. In order to protect hibernating bats effectively, more knowledge about mortality factors is needed. During the winters of 2003–2015, we found 214 dead bats in 12 hibernacula in The Netherlands province of Zuid-Holland. Most bat remains were found in December and January, with a second peak in April. Their remains showed a typical pattern of lesions consistent with those caused by predation by the wood mouse (Apodemus sylvaticus). Trail camera surveys showed that wood mice actively searched for bats. Predation pressure seemed to vary between winters, with a peak in the winters of 2004, 2011 and 2015. The annual mortality (relative to the maximum winter population size) caused by wood mouse predation varied between 0.1 and 8.8 %, with a maximum local effect of 83.6 %. The years with high wood mouse predation pressure were characterized by a long frost period and a low mast production of common oak in the preceding autumn. The size of a hibernaculum and the population density of its bats had an effect on predation-dependent mortality. The highest predation risk occurred near the entrance of bunkers. From these results we tentatively conclude that predation is not incidental and that wood mice actively search for and kill hibernating bats or scavenge for weakened individuals.
Similar content being viewed by others
Introduction
Predation is considered a nearly universal pressure affecting individual animals and has, through evolutionary history, shaped the morphology, behavior and life history traits of prey species. Since bats are very fast in flight, predation pressure on bat populations is likely to be a minor cause of mortality. Indeed, bats usually consist of less than 1 % of the prey of owls (Ruprecht 1979; Mikkola 1983; Kasprzyk et al. 2004). Nonetheless, large aggregations of bats may provide a viable resource for predators (e.g., raptors and owls) and may even become the dominant prey, comprising up to 39 % of the diet of individual predators (Julian and Altringham 1994; Lesinski et al. 2009; Sommer et al. 2009). Since bats and their roosts are protected by European legislation, large aggregations of bats are often found in protected sites. Although these sites may attract flying and non-flying predators and become high-risk areas, studies on predation of bats from this perspective are very limited.
Underground structures and their immediate surroundings often serve as mating sites, hibernacula, and sometimes even maternity roosts for bats. In these areas, bats are frequently reported among the mammalian prey of owl species during summer and autumn, such as barn owl, Tyto alba (Forster and Fairley 1975; Ruprecht 1979, Mertens 1997; Sommer et al. 2009) and tawny owl, Strix aluco (Rackow 1992; Kowalski and Lesiński 1990; Lesiński et al. 2009). Other occasional scavengers and hunters include the otter, Lutra lutra (Forman et al. 2004), kestrel, Falco tinnunculus (Negro et al. 1992) and hobby, Falco subbuteo (Speakman 1991; Fenton et al. 1994). Observations of predators hunting bats in winter roosts are surprisingly scarce. Torpid bats are vulnerable to scavengers and predators. During winter, uninterrupted torpor bouts can last 2 weeks (Daan 1972; Park et al. 2000) and sometimes up to 14 weeks (Harmata 1985). Known predators of hibernating bats include great tit, Parus major (Bogdanowicz 1990; Radzicki et al. 1999; Estók et al. 2010), stone marten, Martes foina (Bekker 1989; Romanowski and Lesiński 1991; Kokurewicz 2004), red fox, Vulpes vulpes (Kokurewicz 2004), and wood mouse, Apodemus sylvaticus (Bekker and Mostert 1991; Mostert 1996).
In order to manage underground structures registered as protected sites for bats effectively, predation risk should be quantitatively assessed at those sites. In this study, we surveyed for, and collected the remains of, predated bats in 12 hibernacula systems to investigate the effect of predation on hibernating bats. This paper has five objectives: (1) to identify the predator; (2) to analyse the frequency and seasonality of predation; (3) to identify the characteristics of a hibernaculum that could increase the risk of predation; (4) to test whether bats show an anti-predator behavioural response; and (5) to estimate the annual mortality caused by predators in The Netherlands during various winters.
Materials and methods
The study was conducted in the province of Zuid-Holland, between the cities of Den Haag (The Hague), Leiden and the town of Wassenaar (between 52°07′ and 52°09′N, 4°18′ and 4°21′E, Fig. 1). The study site consisted of 12 bunker complexes. These belong to the “Atlantic Wall”, an extensive system of coastal fortifications built by the German army during World War II (Saunders 2001). After the war the fortifications were abandoned and were gradually overgrown by vegetation such as European beachgrass, Ammophila arenaria, sea buckthorn, Hippophae rhamnoides, European black elderberry, Sambucus nigra, common oak, Quercus robur, and European Spindle-Tree, Euonymus europaeus. All bunkers are closed to the public by a steel door, with one or two entrances (14 × 20 cm) for bats. Mice can enter freely through small gaps to the side of the doorframe. The bunkers vary in size, underground layout, and the number of entrances. All have underground corridors made of brick connected to one or several concrete bunkers. The corridors are typically approximately 2.0 m high and 1.2 m wide, with an arched ceiling. The size and shape of the bunkers vary according to their original function (e.g., command post, troop quarters, ammunition storage, and garage for searchlight). The first records of bats occupying the bunkers in winter date from the early 1980s (A.-J. Haarsma, unpublished data). The ceilings and walls of both the bunkers and the corridors have numerous crevices and cavities suitable for hibernating bats. Bats also hibernate in the sealed ventilation shafts and metal pipework.
Identifying potential predators
In the winter of 2002 we observed a wood mouse eating a recently deceased bat. Thereafter, remains of partially eaten bats have been collected and inspected in the laboratory. In order to identify the cause of death a preliminary investigation into the possible predators (winter 2009–2010) was conducted. We placed one trail camera (Bushnell, Trophy camera, model 119425C) and monitored the presence and activity of non-flying mammals in the hibernaculum. Simultaneously, we searched all hibernation sites for feces and tracks of potential predators.
Survey method
The hibernacula were surveyed between autumn 2003 and spring 2015 at 1 month intervals from September to April. In this paper, the period between September and April is defined as the winter of the year starting in January. All roosts were systematically inspected for live bats and fresh remains (i.e., not skeletons). All bats were classified in two groups, according to their hanging position: hidden (e.g., in a crevice or ventilation shaft) or free (e.g., hanging from the ceiling or against a wall). Bats which had not died of predation were excluded from the analysis. Some bats may have been undetectable to the observers; however, the sampling error does not vary with time and roost.
Characteristics of hibernacula
The following characteristics were used to describe the hibernacula: maximum distance from the entrance, scale indicating the number of crevices, and vegetation near the entrance. The total length of the studied complexes ranged between 17 and 870 m. However, several complexes have more than one entrance, and entrances are sometimes located halfway down a corridor. We assumed that predators take the shortest route from the entrance to their prey. Therefore, we calculated the maximum distance (underground) from the entrance to the rear of each bunker. Because it was impossible to count the number of crevices suitable for bats, we estimated the overall abundance of crevices using a relative scale from 1 to 100, where 1 meant no crevices and 100 meant that the entire site is full of potential hiding places (this scale does not include ventilation shafts).
Vegetation near the entrance of hibernacula
We measured the shrub and tree cover, expressed in square meters, within a radius of 75 m from the main entrance (maximum 17,671 m2), which coincides with the average home range of a wood mouse. Several studies (Selva et al. 2012; Kleef and Wijsman 2015) have shown that tree masting can influence rodent population cycles, as seed crops of deciduous trees are an important food source for small rodents (such as wood mice) during spring and winter. We used annual data on the seed production of common oak collected by the ‘Vereniging Wildbeheer Veluwe’ in the province of Gelderland as a measure for the availability of acorns in our study area. A review of the available literature shows that the largest seed crops of oaks occurred more of less synchronously in northern Europe (Pucek et al. 1993). The Veluwe data cover the mast production from 2003 to 2015, expressed in kilograms × 106.
Weather data
We retrieved weather data from the European Climate Assessment and Dataset (ECA&D) project (http://www.eca.knmi.nl). We used the data covering the winters from October 2003 to March 2015 gathered by the Royal Netherlands Meteorological Institute. The dataset consisted of daily records of minimum and maximum temperature (in degrees Celsius). Using this dataset, we calculated the number of frost days (a period of 24 h in which the minimum temperature is below 0 °C (32 °F)) and ice days (a period of 24 h in which the temperature remains below freezing all day). These variables were used as indices of the severity of the winter.
Recognition of predator
The remains that we found showed the typical pattern of lesions attributable to predation by wood mice. The skin of the victims is scraped clean. In the process of eating all the soft tissue, the skin is turned inside out, including the skin around the skull and hind legs (Fig. 2a–d). In fresh remains, marks made by the incisors can clearly be seen in patches of skin. The skull is always broken and sometimes completely gone. The brains are eaten starting from the back of the head. Most of the smaller bones and tendons are eaten or bitten through. Wing membranes and parts of the forearm appear to be least digestible and were often left intact. Generally, the upper arms were bitten through, whereas the forearms were left intact.
Typical prey remains of a wood mouse. The skin of the bat is turned inside out. The bones and tendons of legs and upper arms are bitten through. The skull is broken and the skin around the skull is turned inside out. a, b The remains of a Daubenton’s bat; c, d a pond bat (museum voucher numbers NMR999000003397, NMR999000003398, NMR999000003399 and NMR999000003400)
Identification of remains
Bat species found hibernating in the study sites were the pond bat, Myotis dasycneme, Daubenton’s bat, M. daubentonii, whiskered bat, M. mystacinus, Natterer’s bat, M. nattereri, and brown long-eared bat, Plecotus auritus. Recognizable parts of the remains were forearm, hind legs, and fur. Evidence of fungal infection was cleaned prior to identification. Identification was possible by comparing the remains with bats in the collection of the National Museum of Natural History (nowadays Naturalis Biodiversity Center) in Leiden, The Netherlands. Age and sex of the bats could not determined. Although we often found jawbones, we did not use tooth wear patterns to estimate the age of bats, as the reliability of this method is debated (Brunet-Rossinni and Wilkinson 2009). A selection of the collected remains were preserved as vouchers and deposited in the National History Museum Rotterdam, The Netherlands.
Data analyses
To calculate during which conditions wood mice tended to predate bats more often, we used a generalized linear model (GLM) with a Poisson distribution. The number of frost days was used to describe the severity of the winter; mast production of common oak was used as an indicator of food availability.
A Spearman’s rank correlation was used to examine the relationship between the variable pairs: monthly winter population and number of predated bats; hidden winter population (the number of bats in crevices relative to the total winter population) and crevice scale; and hidden winter population and yearly mortality per site. To calculate the relationship between the characteristics of a hibernaculum that appear to increase predation risk (expressed as the total number of predated bats), we used a GLM with a Poisson distribution. Independent variables describing each site were: maximum distance from entrance, scale describing the number of crevices, vegetation near the entrance and winter population size in each site. The significance of each term was tested by excluding it from the model and comparing the resulting change in deviance to a Chi square distribution. Non-significant interaction terms were excluded from the model. We used SPSS 17 for these statistical analyses.
Results
Mammals in hibernacula
The wood mouse was the most commonly observed non-flying mammal species in the winter of 2009–2010 (94 % of the observations). The only other mammal species observed with the trail camera was the brown rat (Rattus norvegicus). Similar results were obtained by studying tracks and feces (Fig. 3). In addition, we found the remains of one common vole (Microtus arvalis).
With the trail camera we were able to prove that wood mice actively searched for prey (Fig. 4a, b). Frequently observed behavior patterns were perching on hind legs and sniffing the air above them, especially near places with hibernating bats. Although we observed mice climbing structures with hibernating bats, we did not observe a wood mouse grabbing and killing a bat.
Observations on predation
Between September 2003 and April 2015 the total number of bats in the 12 hibernacula fluctuated between 699 and 1277 individuals. The dominant species were Daubenton’s bat with 65.2 %, and pond bat with about 30.5 %. Natterer’s bat (0.8 %), whiskered bat (1.2 %), and brown long-eared bat (2.3 %) were found only sporadically. During each winter the number of live bats increased and decreased following a similar pattern with the highest number of individuals always found in December and January.
We found a total of 214 remains of predated bats during the 12 years (16 pond bats and 198 Daubenton’s bats). We also found one dead brown long-eared bat. Since that species only visited our study sites during 3 out of 8 months in winter, this specimen was not included in the analyses. We found nine intact dead bats. These were not included in our analyses, because they died of other causes.
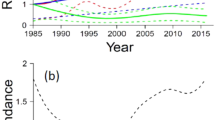
In the 12 years of our study, 247 ringed pond bats were recovered in these hibernacula, five of which had been predated by wood mice (i.e., 2.0 % of the marked population, Fig. 2d). The yearly percentage of predated bats among the maximum surveyed winter population varied between 0.1 and 8.8 % (Fig. 5). In the winters of 2004, 2011 and 2015, the number of predated bats peaked with 38 (5.4 % of the population), 84 (8.8 %) and 41 (3.2 %) predated individuals, respectively. The effect of predation in individual sites was even higher, with a maximum of 83.6 % of the local population of 73 animals during one winter.
Weather extremes
The number of frost days per winter ranged between 18 and 75, the number of ice days ranged between 0 and 20. The winter of 2011, in which the highest number of predated bats was found, was coldest (average +4.7 °C, 75 frost days and 15 ice days), while the winter of 2014 was mildest (average +7.5 °C, 21 frost days and 0 ice day). The two winters (2004 and 2015) in which the second and the third highest number of predated bats were found, were not exceptionally long or cold. The winter of 2004 was mild (only 59 frost days and 1 ice day), with an average temperature of +5.5 °C. The winter of 2015 was warm (with only 47 frost days and 1 ice day), with an average temperature of +6.6 °C.
Effect of weather and acorn production
We analyzed the relationship between the number of predated bats per year and one variable describing the severity of the winter (frost days) and the mast production of the common oak. Both the number of frost days and acorn production contributed to explaining the variation in predation (Table 1). On average, predation was more likely to occur in years with both a high number of frost days and a low production of acorns.
Seasonality of predation
In the first 3 months of the winter, mortality rate was low. In December and January, the wintering population reached its maximum. However, the monthly mortality rate remained low: between 0.1 and 2.7 % of the population. In April, when the wintering population decreased, the mean number of dead bats reached its maximum (Fig. 6). We found a significant (r s = −0.360, P = 0.026, df = 34) negative relationship between the monthly winter population size of living bats and the number of deaths. These results indicate that wood mice do not actively select sites with a high density of bats.
Effects of characteristics of the hibernacula
Crevice scales varied between 5 and 90, with an average of 27.7. We did not find a significant relationship between the hidden winter population (the number of bats hidden in crevices relative to the total winter population) and the crevice scale (r s = 0.358, P = 0.11, df = 10), hence sites with more crevices did not yield more hibernating bats. However, the hidden winter population was significantly negatively correlated with mortality by predation (r s = −0.236, P = 0.07, df = 130). In sites with a high number of hidden bats, mortality by predation was lower than expected.
Sea buckthorn was always found within the 75 m radius around the main entrance. Shrub and tree cover varied from 5 to 96 % of the plot area. Vegetation near the entrance had no significant effect on the predation risk (expressed as the number of predated bats) per site (Table 2). Although the two variables, ‘maximum distance from the entrance’ and ‘average size of the winter population’ were significant predictors for this model (Table 2), the interaction between these two variables was also significant, making it impossible to distinguish which variable was the most important. Although ‘maximum distance from the entrance’ yielded a higher predation risk, the highest number of predated individuals was found at or near the entrance of the bunkers, often within 30 m of the entrance (Fig. 7).
Discussion
Between the winters of 2003 and 2015, we found 214 bats killed by predators in 12 hibernacula in the province of Zuid-Holland. Judging by the typical pattern of lesions, their deaths were the result of predation by wood mice. In a similar study in the province of Gelderland, The Netherlands, a total of more than 300 bat remains were found in the period of 1987–2013 (R. Kaal, unpublished data). Since these data were collected only once per winter (and not during all winters), they were not included in the analysis. In this area the number of predated bats varied from 1 to 120 individuals per winter (9 sites, maximum 24.3 % of the local hibernating population). The high numbers of predated bats, both in Zuid-Holland and in Gelderland, show that predation of bats by wood mice is more common than is generally assumed and the effects of predation in hibernacula may be significant. We further suspect that the effect of predation is more severe in isolated sites than in clustered sites, as isolated sites may take longer to become (re)colonized by bats.
Although wood mice are generally considered scavengers (Montgomery and Montgomery 1990), our study reveals that predation on hibernating bats was not incidental, but that wood mice actively searched for bats (Fig. 4). Mouse-related mortality was estimated to kill between 5.4 and 8.8 % of the hibernating population in years with high predation. This may be an underestimation, as mice tend to take their prey to concealed and safe places. Another factor causing underestimation of the actual numbers of predated bats may be fungal decay. In the humid conditions of the hibernacula, remains of dead bats tend to decay rather quickly.
Unlike other authors (e.g., Julian and Altringham 1994; Petrželková and Zukal 2003; Sommer et al. 2009), who found that high predation mainly occurs at sites with high population densities, our results indicate that population density is only one of the factors influencing predation risk by wood mice. In addition, we found that large sites constitute a higher predation risk. In our study, with only 12 sites, we found no significant effect of the vegetation near the entrance of the hibernacula. Further research is needed, as wood mouse densities differ between woodland and other types of habitat (Tellería et al. 1991; Todd et al. 2000).
The seasonality of mortality was rather similar to that found in other studies (Kokurewicz 2004; Ruczyński et al. 2005). We found a peak in predation-dependent mortality in April. In other hibernacula timing of peak mortality may differ due to differences in predator species, climate (e.g., maritime climate versus continental climate) or other local conditions (such as the size of hibernacula and vegetation type near the entrance).
An increase in mortality of hibernating bats at the end of the winter is often explained by exhaustion of fat reserves or a weakened physical condition as a result of progressing disease (Luis and Hudson 2006). Our study suggests that most deaths at the end of the winter are caused by predators; occupation of more exposed hibernation positions of weakened may increase the risk of predation. Moreover, bats tend to move progressively closer the entrance of a hibernaculum towards the end of the winter (Daan and Wichers 1968; Siivonen and Wermundsen 2008), further increasing their susceptibility to predation.
Winters near the coast of The Netherlands are generally mild, with an average temperature of 5.9 °C across the entire winter period. During mild winter weather, bats are easily disturbed (Haarsma and de Hullu 2012) and as a consequence tend to use more energy than during (mild) frost periods. Thus, in terms of fitness, Dutch bats may benefit from more severe winters. Hence, we suspect that the increase in wood mouse predation during colder winters following a low mast production of common oak is caused by higher energy demands and greater difficulty to find food for wood mice.
By hiding deep in crevices bats can reduce their risk of an encounter with a predator (Barclay and Harder 2003). Although this general behavior may be associated with predator avoidance, bats often appear to lack a perception of risk (Lima and O’Keefe 2013). Bats show little to no behavioral reaction to sound and scent associated with owls and mammalian carnivores (Kalcounis and Brigham 1994; Driessens and Siemers 2010). Some studies (Bekker and Mostert 1991; Ruczyński et al. 2005) postulate that hiding, or using concealed resting positions, can prevent predation. However, we found no relationship between the proportion of hiding bats and the availability of crevices, and our results indicate that the chance of being predated is not affected by the number of available crevices in a hibernaculum. We think that all bats, both in crevices and along the wall, can be predated as long as wood mice can reach those places. In this context, we note that other mammalian/reptilian predators of bats are larger and will not be able to reach bats in crevices.
Consequences for conservation
In order to estimate the total effect of predation by wood mice on bats, we extrapolated our results to all (known) comparable sites in The Netherlands. A selection of more than 1500 of these sites, such as bunkers, ice cellars and other man-made objects, are surveyed annually during the National Winter Census Program of the Dutch Mammal Society. Here, a total of 14,000 bats (on average 9.3 bats per site) are counted each year. In our study area, an estimated average of 1.8 % of the population, i.e., 252 bats, is predated each year. In our study, one sites out of 12 had an increased predation risk of up to 83.6 % during extreme winters. If we again extrapolate this over all sites, we surmise that about 1162 bats hibernate in high-risk sites (125 sites × 9.3 bats). In winters with a long frost period, wood mice may account for an estimated 972 predated bats in these sites.
Bats have a slow reproduction rate (Barclay and Harder 2003). Hence, population numbers are easily affected by high predation pressure. Special care should be taken to protect isolated sites, as disturbances at such sites constitute a greater risk to the viability of local bat populations. Although wood mice are skilled climbers and jumpers, it is possible to prevent them from entering a hibernaculum. Most bunkers in The Netherlands are locked by an iron door with a small opening for bats. Other entrances, such as gaps around the door or a mouse hole, can be closed off. However, adjustments to the entrance with a negative effect on the microclimate of hibernacula, such as limiting the amount of air circulation, should be avoided (Pugh and Altringham 2005; Baranauskas 2006; Kervyn et al. 2009). Beside adaptation to the entrance, adjustments to the interior can decrease predation risk. Unsafe cracks and crevices near the ground can be closed. Shelves along the wall within jumping range of mice (<1.5 m) can be removed. Furthermore, man-made hiding places for bats, such as wood-wool cement boards and hollow building bricks, can be placed along walls out of reach of the mice.
References
Baranauskas K (2006) Bat species composition and abundance in two underground hibernaculae in Vilnius before and after fencing. Ecologija (Vilnius) 1:10–15
Barclay RM, Harder D (2003) Life histories of bats: life in the slow lane. In: Kunz TH, Fenton MB (eds) Bat ecology. University of Chicago Press, Chicago, pp 209–253
Bekker JP (1989) Pine marten, bats and underground limestone mines. SOK Med 13:33–43 (in Dutch with English abstract)
Bekker JP, Mostert K (1991) Predation on bats in The Netherlands: facts and assumptions. Myotis 29:91–96
Bogdanowicz W (1990) Geographic variation and taxonomy of Daubenton’s bat, Myotis daubentonii, in Europe. J Mammal 71:205–218
Brunet-Rossinni AK, Wilkinson GS (2009) Methods for age estimation and the study of senescence in bats. In: Kunz T, Parsons S (eds) Ecological and behavioral methods for the study of bats. Johns Hopkins University Press, Baltimore, pp 315–325
Daan S (1972) Activity during natural hibernation in three species of Vespertilionid bats. Neth J Zool 23:1–71
Daan S, Wichers HJ (1968) Habitat selection of bats hibernating in a limestone cave. Z Säugetierkd 33:262–287
Driessens T, Siemers BM (2010) Cave-dwelling bats do not avoid TMT and 2-PT—components of predator odour that induce fear in other small mammals. J Exp Biol 213:2453–2460
Estók P, Zsebők S, Siemers BM (2010) Great tits search for, capture, kill and eat hibernating bats. Biol Letters 23:59–62
Fenton MB, Rautenbach IL, Smith SE, Swanepoel SM, Grosell J, Van Jaarsveld J (1994) Raptors and bats: threats and opportunities. Anim Behav 48:9–18
Forman DW, Liles G, Barber P (2004) Evidence of lesser horseshoe bat (Rhinolophus hipposideros) predation by otter (Lutra lutra) in a Welsh cave system. Lutra 47:53–56
Forster R, Fairley JS (1975) Further data on barn owls feeding on bank voles, and a new county record of the lesser horse-shoe bat. Ir Nat J 18:251–252
Haarsma A-J, de Hullu E (2012) Keeping bats cool in the winter: hibernating bats and their exposure to ‘hot’ incandescent lamplight. Wildl Biol 18:14–23
Harmata W (1985) The length of awakening time from hibernation of three species of bats. Acta Theriol 30:321–323
Julian S, Altringham JD (1994) Bat predation by a Tawny owl. Naturalist 119:49–56
Kalcounis MC, Brigham RM (1994) Impact of predation risk on emergence by little brown bats, Myotis lucifugus (Chiroptera: Vespertilionidae), from a maternity colony. Ethol 98:201–209
Kasprzyk K, Kitowski I, Czochra K, Krawczyk R (2004) Bats in the diet of owls from the southern part of the Lublin region (SE Poland). Myotis 41–42:75–80
Kervyn T, Lamotte S, Nyssen P, Verschuren J (2009) Major decline of bat abundance and diversity during the last 50 years in southern Belgium. Belg J Zool 139:124–132
Kleef HL, Wijsman HJ (2015) Mast, mice and pine marten (Martes martes): the pine marten’s reproductive response to wood mouse (Apodemus sylvaticus) fluctuations in The Netherlands. Lutra 58:23–33
Kokurewicz T (2004) Sex and age related habitat selection and mass dynamics of Daubenton’s bats Myotis daubentonii (Kuhl 1817) hibernating in natural conditions. Acta Chiropterol 6:121–144
Kowalski M, Lesiński G (1990) The food of the tawny owl (Strix aluco) from near a bat cave in Poland. Bonner Zool Beitr 41:23–26
Lesiński G, Ignaczak M, Manias J (2009) Opportunistic predation on bats by the tawny owl Strix aluco. Anim Biol 59:283–288
Lima SL, O’Keefe JM (2013) Do predators influence the behaviour of bats? Biol Rev 88:626–644
Luis AD, Hudson PJ (2006) Hibernation patterns in mammals: a role for bacterial growth? Funct Ecol 20:471–477
Mertens F (1997) Predation of serotine bat by barn owl. Vlen Nieuwsbrief 29:2–3 (in Dutch)
Mikkola H (1983) Owls of Europe. T & AD Poyser, London
Montgomery SSJ, Montgomery WI (1990) Intrapopulation variation in the diet of the wood mouse Apodemus sylvaticus. J Zool 222:641–651
Mostert K (1996) A wood mouse eats a Daubenton’s bat. Zoogdier 7:33 (in Dutch)
Negro JJ, Ibáñez C, Pérezjordá JL, Delariva MJ (1992) Winter predation by common kestrel Falco tinnunculus on Pipistrelle bats Pipistrellus pipistrellus in southern Spain. Bird Study 39:195–199
Park PJ, Jones G, Ransome RD (2000) Torpor, arousal and activity of hibernating greater horseshoe bats (Rhinolophus ferrumequinum). Funct Ecol 14:580–588
Petrželková KJ, Zukal J (2003) Does a live barn owl (Tyto alba) affect emergence behaviour of serotine bats (Eptesicus serotinus)? Acta Chiropterol 5:177–184
Pucek Z, Jędrzejewski W, Jędrzejewska B, Pucek M (1993) Rodent population dynamics in a primeval deciduous forest (Białowieża National Park) in relation to weather, seed crop, and predation. Acta Theriol 38:199–232
Pugh M, Altringham JD (2005) The effect of gates on cave entry by swarming bats. Acta Chiropterol 7:293–299
Rackow W (1992) Wasserfledermaus (Myotis daubentonii) verfolgt Waldkauz (Strix aluco). Nyctalus 4:538–539 (in German with English abstract)
Radzicki G, Hejduk J, Bañbura J (1999) Tits (Parus major and Parus caeruleus) preying upon hibernating bats. Ornis Fennica 76:93–94
Romanowski J, Lesiński G (1991) A note on the diet of stone marten in southeastern Romania. Acta Theriol 36:201–204
Ruczyński I, Ruczyńska I, Kasprzyk K (2005) Winter mortality rates of bats inhabiting man-made shelters (northern Poland). Acta Theriol 50:161–166
Ruprecht A (1979) Bats (Chiroptera) as constituents of the food of barn owls Tyto alba in Poland. Ibis 121:489–494
Saunders A (2001) Hitler’s Atlantic wall. Sutton Publishing, Stroud
Selva N, Hobson KA, Cortés-Avizanda A, Zalewski A, Donázar JA (2012) Mast pulses shape trophic interactions between fluctuating rodent populations in a primeval forest. PLoS One 7(12):e51267
Siivonen Y, Wermundsen T (2008) Characteristics of winter roosts of bat species in southern Finland. Mammalia 72:50–56
Sommer RS, Niederle M, Labes R, Zoller H (2009) Bat predation by the barn owl Tyto alba in a hibernation site of bats. Folia Zool 58:98–103
Speakman JR (1991) The impact of predation by birds on bat populations in the British Isles. Mammal Rev 21:123–142
Tellería JL, Santos T, Alcántara M (1991) Abundance and food-searching intensity of wood mice (Apodemus sylvaticus) in fragmented forests. J Mammal 72:183–187
Todd IA, Tew TE, Macdonald DW (2000) Arable habitat use by wood mice (Apodemus sylvaticus). 1. Macrohabitat. J Zool 250:299–303
Acknowledgments
This research project was supported by the Dutch government (Ministry for Agriculture, Fisheries and Nature Conservation). The Dutch Mammal Society provided data gathered for their National Winter Census Program. We thank all managers and owners of our 12 study sites, Dunea (Water Utility Company), Staatsbosbeheer (State Forest Service) and the Ministry of Defence, for their cooperation and for access to these hibernacula. Thanks are due to all the people who helped with the fieldwork, especially Bart Noort, Gerben Achterkamp and Carolien van der Graaf. We also wish to thank Chris Smeenk, Takashi Saitoh and Jaap van Schaik for their constructive criticism and valuable suggestions for this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Haarsma, AJ., Kaal, R. Predation of wood mice (Apodemus sylvaticus) on hibernating bats. Popul Ecol 58, 567–576 (2016). https://doi.org/10.1007/s10144-016-0557-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10144-016-0557-y