Abstract
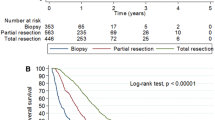
The incidence of glioblastoma (GBM) has increased in patients aged 70 years or older, and will continue to grow. Elderly GBM patients have been excluded from most clinical trials; furthermore, optimal care management as well as benefit/risk ratio of GBM treatments are still being debated. This study describes oncological patterns of care, prognostic factors, and survival for patients ≥70 years in France. We identified patients over 70 with newly diagnosed and histologically confirmed GBM on data previously published by the French Brain Tumor DataBase. We included 265 patients. Neurological deficits and mental status disorders were the most frequent symptoms. The surgery consisted of resection (RS n = 95) or biopsy (B n = 170); 98 patients did not have subsequent oncological treatment. After surgery, first-line treatment consisted of radiotherapy (RT n = 76), chemotherapy (CT n = 52), and concomitant radiochemotherapy (CRC n = 39). The median age at diagnosis was 76, 74, and 73 years, respectively, for the untreated, B + RT and/or CT, RS ± RT and/or CT groups. Median survival (in days, 95 % CI) with these main strategies, when analyzed according to surgical groups, was: B-CT n = 41, 199[155–280]; B-CRC n = 21, 318[166–480]; B-RT n = 37, 149[130–214]; RS-CT n = 11, 245[211–na]; RS-CRC n = 18, 372[349–593]; RS-RT n = 39, 269[218–343]. This population study for elderly GBM patients is one of the most important in Europe, and could be considered as a historical cohort to compare future treatments. Moreover, we can hypothesize that elderly patients (versus patients <70 years) are undertreated. Karnofsky performance status seems to be the most relevant clinical predictive factor, and RS and CRC have a positive impact on survival for elderly GBM patients in the general population, at least when feasible.


Similar content being viewed by others
References
Barker CA, Chang M, Chou JF, Zhang Z, Beal K, Gutin PH, Iwamoto FM (2012) Radiotherapy and concomitant temozolomide may improve survival of elderly patients with glioblastoma. J Neurooncol 109:391–397. doi:10.1007/s11060-012-0906-4
Barnholtz-Sloan JS, Williams VL, Maldonado JL, Shahani D, Stockwell HG, Chamberlain M, Sloan AE (2008) Patterns of care and outcomes among elderly individuals with primary malignant astrocytoma. J Neurosurg 108:642–648. doi:10.3171/jns/2008/108/4/0642
Bauchet L, Mathieu-Daude H, Fabbro-Peray P, Rigau V, Fabbro M, Chinot O, Pallusseau L, Carnin C, Laine K, Schlama A, Thiebaut A, Patru MC, Bauchet F, Lionnet M, Wager M, Faillot T, Taillandier L, Figarella-Branger D, Capelle L, Loiseau H, Frappaz D, Campello C, Kerr C, Duffau H, Reme-Saumon M, Tretarre B, Daures JP, Henin D, Labrousse F, Menei P, Honnorat J (2010) Oncological patterns of care and outcome for 952 patients with newly diagnosed glioblastoma in 2004. Neuro Oncol 12:725–735. doi:10.1093/neuonc/noq030
Bauchet L, Rigau V, Mathieu-Daudé H, Fabbro-Peray P, Palenzuela G, Figarella-Branger D, Moritz J, Puget S, Bauchet F, Pallusseau L, Duffau H, Coubes P, Trétarre B, Labrousse F, Dhellemmes P (2009) Clinical epidemiology for childhood primary central nervous system tumors. J Neurooncol 92:87–98. doi:10.1007/s11060-008-9740-0
Bauchet L, Rigau V, Mathieu-Daudé H, Figarella-Branger D, Hugues D, Palusseau L, Bauchet F, Fabbro M, Campello C, Capelle L, Durand A, Trétarre B, Frappaz D, Henin D, Menei P, Honnorat J, Segnarbieux F (2007) French brain tumor data bank: Methodology and first results on 10,000 cases. J Neurooncol 84:189–199. doi:10.1007/s11060-007-9356-9
Brandes AA, Franceschi E, Tosoni A, Benevento F, Scopece L, Mazzocchi V, Bacci A, Agati R, Calbucci F, Ermani M (2009) Temozolomide concomitant and adjuvant to radiotherapy in elderly patients with glioblastoma. Cancer 115:3512–3518. doi:10.1002/cncr.24406
Central Brain Tumor Registry of the United States (CBTRUS) CBTRUS 2012 Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2004–2008 http://www.cbtrus.org/2012-NPCR-SEER/CBTRUS_Report_2004-2008_3-23-2012.pdf. Revised March 23, 2012. Accessed 06 Nov 2012
Chargari C, Feuvret L, Bauduceau O, Ricard D, Cuenca X, Delattre J-Y, Mazeron J-J (2012) Treatment of elderly patients with glioblastoma: from clinical evidence to molecular highlights. Cancer Treat Rev 38:988–995. doi:10.1016/j.ctrv.2011.12.010
Davis FG, Malmer BS, Aldape K, Barnholtz-Sloan JS, Bondy ML, Brännström T, Bruner JM, Burger PC, Collins VP, Inskip PD, Kruchko C, McCarthy BJ, McLendon RE, Sadetzki S, Tihan T, Wrensch MR, Buffler PA (2008) Issues of diagnostic review in brain tumor studies: from the Brain Tumor Epidemiology Consortium. Cancer Epidemiol Biomark Prev 17:484–489. doi:10.1158/1055-9965.epi-07-0725
Dolecek TA, Propp JM, Stroup NE, Kruchko C (2012) CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2005–2009. Neuro Oncol 14(suppl 5):v1–v49. doi:10.1093/neuonc/nos218
Gállego Perez-Larraya J, Ducray F, Chinot O, Catry-Thomas I, Taillandier L, Guillamo JS, Campello C, Monjour A, Cartalat-Carel S, Barrie M, Huchet A, Beauchesne P, Matta M, Mokhtari K, Tanguy ML, Honnorat J, Delattre JY (2011) Temozolomide in elderly patients with newly diagnosed glioblastoma and poor performance status: an ANOCEF phase II trial. J Clin Oncol 29:3050–3055. doi:10.1200/jco.2011.34.8086
Graus F, Bruna J, Pardo J, Escudero D, Vilas D, Barceló I, Brell M, Pascual C, Crespo JA, Erro E, García-Romero JC, Estela J, Martino J, García-Castaño A, Mata E, Lema M, Gelabert M, Fuentes R, Pérez P, Manzano A, Aguas J, Belenguer A, Simón A, Henríquez I, Murcia M, Vivanco R, Rojas-Marcos I, Muñoz-Carmona D, Navas I, de Andrés P, Mas G, Gil M, Verger E (2013) Patterns of care and outcome for patients with glioblastoma diagnosed during 2008–2010 in Spain. Neuro Oncol [Epub ahead of print]
Gulati S, Jakola AS, Johannesen TB, Solheim O (2012) Survival and Treatment Patterns of glioblastoma in the elderly: a population-based study. World Neurosurg 78:518–526. doi:10.1016/j.wneu.2011.12.008
Hegi ME, Diserens A-C, Gorlia T, Hamou M-F, de Tribolet N, Weller M, Kros JM, Hainfellner JA, Mason W, Mariani L, Bromberg JEC, Hau P, Mirimanoff RO, Cairncross JG, Janzer RC, Stupp R (2005) MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med 352:997–1003. doi:10.1056/NEJMoa043331
Iwamoto FM, Cooper AR, Reiner AS, Nayak L, Abrey LE (2009) Glioblastoma in the elderly. Cancer 115:3758–3766. doi:10.1002/cncr.24413
Iwamoto FM, Reiner AS, Panageas KS, Elkin EB, Abrey LE (2008) Patterns of care in elderly glioblastoma patients. Ann Neurol 64:628–634. doi:10.1002/ana.21521
Johnson DR, O’Neill BP (2012) Glioblastoma survival in the United States before and during the temozolomide era. J Neurooncol 107:359–364. doi:10.1007/s11060-011-0749-4
Keime-Guibert F, Chinot O, Taillandier L, Cartalat-Carel S, Frenay M, Kantor G, Guillamo J-S, Jadaud E, Colin P, Bondiau P-Y, Meneï P, Loiseau H, Bernier V, Honnorat J, Barrié M, Mokhtari K, Mazeron J-J, Bissery A, Delattre J-Y (2007) Radiotherapy for glioblastoma in the elderly. N Engl J Med 356:1527–1535. doi:10.1056/NEJMoa065901
Kleihues P, Cavenee WK (2000) Tumours of the nervous system, pathology and genetics classification of tumours. International Agency for Research on Cancer Press, Lyon
Koshy M, Villano JL, Dolecek TA, Howard A, Mahmood U, Chmura SJ, Weichselbaum RR, McCarthy BJ (2011) Improved survival time trends for glioblastoma using the SEER 17 population-based registries. J Neurooncol 107:207–212. doi:10.1007/s11060-011-0738-7
Malmström A, Grønberg BH, Marosi C, Stupp R, Frappaz D, Schultz H, Abacioglu U, Tavelin B, Lhermitte B, Hegi ME, Rosell J, Henriksson R (2012) Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: the Nordic randomised, phase 3 trial. Lancet Oncol 13:916–926. doi:10.1016/s1470-2045(12)70265-6
Minniti G, Lanzetta G, Scaringi C, Caporello P, Salvati M, Arcella A, De Sanctis V, Giangaspero F, Enrici RM (2012) Phase II study of short-course radiotherapy plus concomitant and adjuvant temozolomide in elderly patients with glioblastoma. Int J Radiat Oncol Biol Phys 83:93–99. doi:10.1016/j.ijrobp.2011.06.1992
Reardon DA (2012) Treatment of elderly patients with glioblastoma. Lancet Oncol 13:656–657. doi:10.1016/S1470-2045(12)70186-9
Reyngold M, Lassman A, Chan T, Yamada Y, Gutin P, Beal K (2012) Abbreviated course of radiation therapy with concurrent temozolomide for high-grade glioma in patients of advanced age or poor functional status. J Neurooncol 110:369–374. doi:10.1007/s11060-012-0972-7
Rigau V, Zouaoui S, Mathieu-Daudé H, Darlix A, Maran A, Trétarre B, Bessaoud F, Bauchet F, Attaoua R, Fabbro-Peray P, Fabbro M, Kerr C, Taillandier L, Duffau H, Figarella-Branger D, Costes V, Bauchet L (2011) French Brain Tumor DataBase: 5-Year histological results on 25 756 cases. Brain Pathol 21:633–644. doi:10.1111/j.1750-3639.2011.00491.x
Rønning PA, Helseth E, Meling TR, Johannesen TB (2012) A population-based study on the effect of temozolomide in the treatment of glioblastoma multiforme. Neuro Oncol 14:1178–1184. doi:10.1093/neuonc/nos153
Scott J, Tsai Y-Y, Chinnaiyan P, Yu H-HM (2011) Effectiveness of radiotherapy for elderly patients with glioblastoma. Int J Radiat Oncol Biol Phys 81:206–210. doi:10.1016/j.ijrobp.2010.04.033
Scott JG, Bauchet L, Fraum TJ, Nayak L, Cooper AR, Chao ST, Suh JH, Vogelbaum MA, Peereboom DM, Zouaoui S, Mathieu-Daudé H, Fabbro-Peray P, Rigau V, Taillandier L, Abrey LE, DeAngelis LM, Shih JH, Iwamoto FM (2012) Recursive partitioning analysis of prognostic factors for glioblastoma patients aged 70 years or older. Cancer 118:5595–5600. doi:10.1002/cncr.27570
Scott JG, Suh JH, Elson P, Barnett GH, Vogelbaum MA, Peereboom DM, Stevens GHJ, Elinzano H, Chao ST (2011) Aggressive treatment is appropriate for glioblastoma multiforme patients 70 years old or older: a retrospective review of 206 cases. Neuro Oncol 13:428–436. doi:10.1093/neuonc/nor005
Stummer W, Pichlmeier U, Meinel T, Wiestler OD, Zanella F, Reulen H-J (2006) Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol 7:392–401. doi:10.1016/S1470-2045(06)70665-9
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJB, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987–996. doi:10.1056/NEJMoa043330
Vuorinen V, Hinkka S, Färkkilä M, Jääskeläinen J (2003) Debulking or biopsy of malignant glioma in elderly people—a randomised study. Acta Neurochir (Wien) 145:5–10. doi:10.1007/s00701-002-1030-6
Westphal M, Hilt DC, Bortey E, Delavault P, Olivares R, Warnke PC, Whittle IR, Jääskeläinen J, Ram Z (2003) A phase 3 trial of local chemotherapy with biodegradable carmustine (BCNU) wafers (Gliadel wafers) in patients with primary malignant glioma. Neuro Oncol 5:79–88, 10.1215/S1522-8517-02-00023-6
Wick W, Platten M, Meisner C, Felsberg J, Tabatabai G, Simon M, Nikkhah G, Papsdorf K, Steinbach JP, Sabel M, Combs SE, Vesper J, Braun C, Meixensberger J, Ketter R, Mayer-Steinacker R, Reifenberger G, Weller M, NOASGotN-oWGotGCS (2012) Temozolomide chemotherapy alone versus radiotherapy alone for malignant astrocytoma in the elderly: the NOA-08 randomised, phase 3 trial. Lancet Oncol 13:707–715. doi:10.1016/s1470-2045(12)70164-x
Wrensch M, Minn Y, Chew T, Bondy M, Berger MS (2002) Epidemiology of primary brain tumors: current concepts and review of the literature. Neuro Oncol 4:278–299. doi:10.1093/neuonc/4.4.278
Zouaoui S, Rigau V, Mathieu-Daudé H, Darlix A, Bessaoud F, Fabbro-Peray P, Bauchet F, Kerr C, Fabbro M, Figarella-Branger D, Taillandier L, Duffau H, Trétarre B, Bauchet L (2012) Recensement national histologique des tumeurs primitives du système nerveux central : résultats généraux sur 40 000 cas, principales applications actuelles et perspectives. Neurochirurgie 58:4–13. doi:10.1016/j.neuchi.2012.01.004
Acknowledgements
First, we would like thank the patients and families. We also thank all pathologists, neurosurgeons, neurologists, oncologists, general practitioners, biostatisticians, clinical research technicians, and all those who participated in this important collaborative work.
Funding
This work was conducted with the financial support of grants from the French Institut National du Cancer (INCa), Ligue Nationale Contre le Cancer, Association des Neuro-Oncologues d’Expression Française, Société Française de Neurochirurgie, Associations pour la Recherche sur les Tumeurs Cérébrales (ARTC and ARTC Sud), Schering-Plough Laboratory, Roche Laboratory, Sophysa Laboratory, Archimedes Pharma Laboratory, Département de l’Hérault, Rotary Club (AGLY), and Groupe de Neuro-Oncologie du Languedoc Roussillon.
Conflict of interest
None declared.
Author information
Authors and Affiliations
Corresponding author
Additional information
Comments
Walter Stummer, Münster, Germany
How old is too old for state-of-the art glioblastoma therapy and what is the consequence for neurosurgeons?
As we are all getting older we are not necessarily feeling older. The world around us is simply getting younger and at some point we fail to understand why patients of age ranges that we will reach sooner than we would prefer, are not receiving treatments we are discussing at our meetings. In addition, it is an interesting fact that in many trials in malignant gliomas we are simply not treating a representative population. In the Stupp trial (Stupp et al. 2005) the median age was only 56 years, in the Gliadel trial (Westphal et al. 2003) median age was 53 years, yet median age in the general population of glioblastoma patients is 63 years (cbtrus.org). Possibly, we are excluding the true population in these studies because it is our inner belief that older patients will not withstand rigorous standard therapy or have an inherent worse prognosis and will dilute our study results. But careful, this may be a self-fulfilling prophecy!
Nowadays, we are accepting the demographic change and are now more and more focusing on older patients and are conceiving trials looking at these patients. Nevertheless, we have interesting ideas about what is “old”. In the recent “elderly” trials in GBM, elderly have been defined to be >65 years in the Methusalem Study (NOA 08; Wick et al. 2012) and 60 years in the Nordic trial (Malmström et al. 2012). The results of these trials have found their way into several guidelines so far. From a surgical point of view, it is unfortunate that resection status, as derived from early post-operative MRI, was not element of these studies. From a surgical perspective, these were missed opportunities to judge whether resection in the “elderly” matters. Studies looking at surgery in the truly elderly are rare. Vuorinen et al. (2003) presented a randomized study testing biopsy against surgery in glioblastoma patients with a median age of 72 years favoring patients with resection. In a cohort of elderly glioblastoma patients with a median age of 70 years, Ewelt et al. (2012) demonstrated extent of resection to be an independent prognostic factor for survival.
Sonai Zouaoui and co-workers here present a cohort of 256 patients with glioblastomas compiled from The French Brain Tumor Database (FBTDB). To be elderly was defined as having a median age beyond 70 years, with a median age of 75 years.
In this analysis, patients receiving surgery and concomitant radiochemotherapy had the best outcomes. These results suggest that in elderly patients maximal therapy should not be simply withheld because of age. Unfortunately, the present analysis lacks multivariate methodology and offers no analysis of factors that were involved in the initial decision to resect or to biopsy. Thus, only the “good” patients (e.g., with high KPS, small, non-eloquently located tumors) may have received maximal therapy and outcome only depended on non-therapeutic prognostic factors. I do however believe that these results cannot be disregarded.
My group has tackled the question of the value of maximal therapy in older patients in some of our studies. In one study—a safety study of 5-ALA—data were collected during the time of transition from simple radiotherapy to concomitant radiochemotherapy after the seminal Stupp-Trial. Thus, we essentially were studying two cohorts, one with radiotherapy and one with radiochemotherapy (Stummer et al. 2011) with median ages at 68 years. We compared these cohorts and stratified by age. Older patients treated with a combination of fluorescence-guided resections and concomitant radiochemotherapy survived 16.3 months and significantly longer than patients treated “only” by surgery with ALA and radiotherapy (11.2 months). Due to the pseudorandomisation afforded by the change in the paradigms of post-operative therapy, the cohorts defined here were well balanced according to the known prognostic factors.
Given the available and emerging data, my conviction is that we should generally not exclude patients from established therapies just because they are old, as suggested by the work of Zouaoui et al. This includes cytoreductive surgery whenever safely possible using established techniques for intra-operative location of tumor, e.g. with Gliolan® or intra-operative MRI, and mapping/monitoring including awake craniotomies, to make surgery as effective and safe as possible.
References
Ewelt C, Goeppert M, Rapp M, Steiger HJ, Stummer W, Sabel M. Glioblastoma multiforme of the elderly: the prognostic effect of resection on survival. J Neurooncol. 2011 Jul;103(3):611–8.
Malmström A, Grønberg BH, Marosi C, Stupp R, Frappaz D, Schultz H, Abacioglu U, Tavelin B, Lhermitte B, Hegi ME, Rosell J, Henriksson R; Nordic Clinical Brain Tumour Study Group (NCBTSG). Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: the Nordic randomized, phase 3 trial. Lancet Oncol. 2012 Sep;13(9):916–26.
Stummer W, Nestler U, Stockhammer F, Krex D, Kern BC, Mehdorn HM, Vince GH, Pichlmeier U. Favorable outcome in the elderly cohort treated by concomitant temozolomide radiochemotherapy in a multicentric phase II safety study of 5-ALA. J Neurooncol. 2011 Jun;103(2):361–70.
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO; European Organisation for Research and Treatment of Cancer Brain Tumor and RadiotherapyGroups; National Cancer Institute of Canada Clinical Trials Group. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005 Mar 10;352(10):987–96.
Wick W, Platten M, Meisner C, Felsberg J, Tabatabai G, Simon M, Nikkhah G, Papsdorf K, Steinbach JP, Sabel M, Combs SE, Vesper J, Braun C, Meixensberger J, Ketter R, Mayer-Steinacker R, Reifenberger G, Weller M; NOA-08 Study Group of Neuro-oncology Working Group (NOA) of German Cancer Society. Temozolomide chemotherapy alone versus radiotherapy alone for malignant astrocytoma in the elderly: the NOA-08 randomized, phase 3 trial. Lancet Oncol. 2012 Jul;13(7):707–15.
Vuorinen V, Hinkka S, Färkkilä M, Jääskeläinen J. Debulking or biopsy of malignant glioma in elderly people—a randomized study. Acta Neurochir (Wien). 2003 Jan;145(1):5–10.
Jürgen Voges, Magdeburg, Germany
The disease glioblastoma multiforme (GBM) has its incidence peak in the sixth through eighth decades of life. With the aging of the population, it is foreseeable that the number of diseased patients will increase in particular in industrial countries. Thus, the here-presented statistical analysis of data of glioblastoma patients ≥ 70 years in age at the time of first diagnosis focused on an important patient subgroup. The tumor data bank utilized by the authors had some shortcomings such as the long delay between data entry and analysis, the lack of biomolecular data, no standardized assessment of the radicality of tumor resection, etc. as mentioned in the manuscript. Nowadays, this analysis demonstrates that already the tenacious sampling of basic data renders possible to characterize the impact of different treatment modalities and prognostic variables on survival. This information will in turn enable researchers to improve the design of prospective clinical studies and to adapt the clinical protocols to relevant problems and questions.
Clearly, more can be expected from “complete” data banks, which to establish and maintain is time consuming and laborious but absolute necessary. In the light of the health economic challenges of the upcoming decades, specific financial support of these activities will be essential. However, the fact that a perfect setting is not realized momentarily should nobody discourage to use already existing tumor networks and or databases intensively.
With the participation of Société Française de Neurochirurgie (SFNC) and the Club de Neuro-Oncologie of the Société Française de Neurochirurgie (CNO-SFNC), Société Française de Neuropathologie (SFNP), and Association des Neuro-Oncologues d’Expression Française (ANOCEF)
Rights and permissions
About this article
Cite this article
Zouaoui, S., Darlix, A., Fabbro-Peray, P. et al. Oncological patterns of care and outcomes for 265 elderly patients with newly diagnosed glioblastoma in France. Neurosurg Rev 37, 415–424 (2014). https://doi.org/10.1007/s10143-014-0528-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10143-014-0528-8