Abstract
Background
Even though indications for endoscopic resection (ER) in early gastric cancer are determined based on the potential risk of lymph node metastasis, the criteria for ER remain controversial. Sentinel node (SN) mapping for early gastric cancer can help determine regional lymphatic flow patterns. The aim of this study was to assess lymphatic flow according to the SN concept in patients with early gastric cancer, especially those who satisfy the expanded criteria for ER.
Methods
We retrospectively enrolled 301 patients diagnosed with pT1 adenocarcinoma who had undergone gastrectomy with SN mapping and had no lymphovascular invasion. Patients were categorized into six groups based on oncological assessment. We analyzed lymphatic flow, including the number of identified SN and SN basin, and the rate of SN metastasis in each group.
Results
Of the 301 patients, 128 (42.5%) met the criteria for ER, with 18 in the absolute group and 110 in the expanded group; 173 (57.5%) were assigned to the surgical group. SN metastasis rate tended to be higher in surgical group patients than in ER criteria patients. In the expanded criteria group, the sub-group of patients with intramucosal, undifferentiated adenocarcinoma measuring 20 mm or less had a significantly greater number of identified SNs (p = 0.013) and SN basins (p = 0.032). Furthermore, SN metastasis was observed only in this group.
Conclusions
Patients with intramucosal, nonulcerated, undifferentiated adenocarcinoma measuring 20 mm or less could develop a lymphatic network. For these patients, careful follow-up is required after ER.
Similar content being viewed by others
Introduction
Early gastric cancer is defined as tumor invasion limited to the mucosal or the submucosal layer, irrespective of lymph node metastasis (LNM) [1], and the treatment approach to early gastric cancer is based on the potential risk of LNM. Endoscopic resection (ER) is deemed useful for tumors that have a very low probability of LNM and are suitable for en bloc resection. Owing to technical advances, ER can be safely used in early gastric cancer and could also help maintain patients’ quality of life after gastric resection. Japanese gastric cancer treatment guidelines provide two independent sets of indications for ER: (1) an absolute indication for standard ER and (2) an expanded indication for ER to be used as an investigational treatment. ER is the standard treatment for a differentiated-type adenocarcinoma without ulcerative findings, with depth of invasion clinically diagnosed as T1a, and a diameter < 2 cm [2]. However, ER criteria in patients who fulfill the criteria for investigational treatment, especially with undifferentiated type of tumor, remain controversial. Although Gotoda et al. [3] have proposed expanded ER criteria that include undifferentiated intramucosal cancer or minute submucosal penetration (Fig. 1), some studies have reported the incidence of LNM in patients who satisfy the expanded criteria. Specifically, Kang et al. [4] have reported that LNM occurred in 15% of the patients with submucosal lesions who met the expanded criteria. Wang et al. [5] have also indicated that the rate of LNM was as high as 8.7% when expanded criteria were used. Furthermore, Oh et al. [6] have suggested that ER may not be a better option in patients with mucosal undifferentiated cancer because undifferentiated-type cancer is significant risk factor of LNM even in mucosal carcinoma. Therefore, ER criteria require further evaluation from a different perspective.
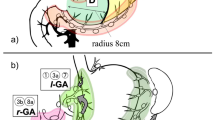
Guidelines for endoscopic resection. Blue marker represents an absolute indication lesion for EMR/ESD, yellow represents an expanded indication lesion for ESD, and gray represents surgical indication. Moreover, upper, middle, and lower rows in each square indicate the number of identified SN, number of identified SN basins (1/2/3/4), and the rate of SN metastasis (95% CI), respectively
Recently, sentinel node (SN) mapping has been applied to early gastric cancer, apart from melanoma [7, 8] and breast cancer [9], and the feasibility of SN mapping, according to the SN concept, has been demonstrated by some facilities, including our institution [10,11,12,13]. Additionally, gastrectomy with SN mapping, called SN navigation surgery, facilitates early recovery and prevents post-gastrectomy issues such as dumping syndrome and weight loss. SN is defined as the first lymph node that receives lymphatic drainage from the primary tumor, and this lymph node is thought to be the first possible site of micrometastasis (MM) in the direction of lymphatic flow from the primary lesion. Therefore, it may be possible to ascertain the status of regional lymphatic flow by evaluating SN pathology [12].
We hypothesized that there is a different status of regional lymphatic flows among the patients with early gastric adenocarcinoma that satisfy ER criteria. These findings would facilitate in considering the indication of endoscopic treatment. Thus, the aim of this study was to assess lymphatic flow during SN mapping in patients with early gastric cancer, especially those meeting expanded ER criteria. We think that the findings can help determine appropriate treatment strategies for early gastric cancer.
Materials and methods
Patients
We retrospectively enrolled 495 patients who were diagnosed with single-lesion cT1N0M0 gastric cancer and had undergone gastric surgery with SN mapping as the primary treatment for gastric cancer at the Keio University, Tokyo, Japan, between November 1999 and December 2017. From these, we identified 301 patients who were diagnosed as pT1 with no lymphovascular invasion or identified SN. We excluded patients who had undergone ER as primary treatment because the validity and accuracy of SN biopsy after endoscopic treatment has not been investigated fully [14]. Additionally, 2 patients in whom the SN remained undetected were excluded.
Prior to treatment, patients were evaluated by upper gastrointestinal endoscopy, thoracic abdominal computed tomography, laboratory tests, and gastrography. Clinical cancer stage was determined according to Japanese gastric cancer treatment guidelines (4th edition) [2] and TNM classification [15]. Histological grading was performed according to the Japanese Classification for Gastric Cancer [16]. Poor carcinoma, signet-ring cell carcinoma, and mucinous carcinoma were defined as undifferentiated adenocarcinoma, whereas papillary and tubular adenocarcinoma were defined as differentiated adenocarcinoma. In cases of mixed type tumors, histology was defined by the predominant component. Detailed D2–40 staining was performed for the diagnosis of lymphovascular invasion, and Elastica van Gieson (EVG) staining was performed for the diagnosis of detailed vascular invasion diagnosis.
Based on oncological assessment, 301 patients were categorized into 6 groups as follows [3] (Fig. 1).
Group 1 (absolute criteria) Intramucosal, differentiated adenocarcinoma, measuring 20 mm or less, nonulcerated.
Group 2 (expanded criteria) Intramucosal, differentiated adenocarcinoma, larger than 20 mm, nonulcerated.
Group 3 (expanded criteria) Intramucosal, differentiated adenocarcinoma, measuring 30 mm or less, ulcerated.
Group 4 (expanded criteria) slight submucosal (<500 μm), differentiated adenocarcinoma, measuring 30 mm or less.
Group 5 (expanded criteria) Intramucosal, undifferentiated adenocarcinoma, measuring 20 mm or less, nonulcerated.
Group 6 (surgical criteria) Other intramucosal or submucosal lesions.
Hospital records were used to retrospectively retrieve clinical and demographic data, including age, gender, and pathological findings. We compared the results of SN mapping among the six groups described above.
SN mapping and surgical procedures
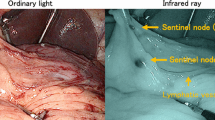
A dual-tracer method that utilized a radioactive colloid and a blue dye to detect SN was used [11]. We endoscopically injected 2 ml (150 MBq) of technetium-99m tin colloid solution into the gastric submucosal layer surrounding the primary tumor in four quadrants using an endoscopic puncture needle 1 day before gastric surgery. Additionally, we intraoperatively injected blue dye (indocyanine green or 1% isosulfan blue) in an identical manner. Within 15 min after the injection of the blue dye, SNs and blue-colored lymphatic vessels could be visually identified [12]. Simultaneously, a hand-held gamma probe (GPS navigator; RMD Instruments, Watertown, MA, USA) was used to detect radioactive SNs. LNs with radioactivity > 10 times the background activity and/or LNs that were stained blue were defined as SNs. The gastric lymphatic compartment was divided into five areas, viz., l-GA basin, including LNs 1, 3a, and 7; r-GA basin, including LNs 3b, 5, and 8a; l-GEA basin, including LNs 4sa and 4sb; r-GEA basin, including LNs 4d and 6; and p-GA basin, including LN 11p [17]. The other LNs surrounding the stomach (LNs 2, 9, 10, 11d, and 14v) were considered part of a sub-basin. The presence of metastasis in SN was diagnosed by intraoperative hematoxylin and eosin (H&E) staining of a cut surface of a frozen section in each SN, considering that the surgical procedure was chosen according to the intraoperative SN mapping results. However, more detailed diagnoses, such as SN macrometastasis, MM, and isolated tumor-cell metastasis, were performed using formalin-fixed specimens postoperatively. According to the TNM classification system, SN macrometastasis was defined as the metastasis of tumor cells measuring ≥ 2 mm in SN, whereas a metastasis of < 2 mm in diameter is regarded as a MM. We also assessed single tumor cells or small clusters of cells measuring < 0.2 mm in their longest dimension that could be detected using routine hematoxylin and eosin (H&E) staining on formalin-fixed specimens [15, 18]. If a more detailed diagnosis is needed, cytokeratin staining is performed.
Depending on the location of the main tumor and the SN basin and their sizes, we performed total, distal, proximal, pylorus-preserving gastrectomy, or local resection. If all SN metastasis were negative, we performed function-preserving procedures such as a local resection. However, in the cases with multiple SN basins, minimized surgery may be difficult to maintain blood flow in the remaining stomach even without SN metastases. For such patients, radical gastrectomy was considered. This study was approved by the institutional review board of the Keio University School of Medicine, and informed consent for the entire procedure of SN mapping was obtained from all patients before surgery.
Statistical analysis
We used the χ2 test to analyze categorical variables and the Mann–Whitney U test to analyze continuous variables. One-way analysis of variance (ANOVA) was used for comparisons among the three parameters of patients’ background. Statistical analyses were performed using Stata/SE 12.1 for Mac (Stata Corp, College Station, TX, USA) and a p value < 0.05 was considered significant.
Results
Background characteristics
We retrospectively enrolled 206 men and 95 women in this study with a mean age of 59.8 ± 11.1 years. Of these patients, 128 (42.5%) met the criteria for ER, with 18 in the absolute group and 110 in the expanded criteria group. Table 1 shows clinicopathological characteristics of the study population. The main tumor location was the middle gastric body (upper, 51; middle, 175; lower, 75), and mean tumor size was 27.5 ± 13.6 mm. Furthermore, 194 (64%) underwent distal gastrectomy, 49 (16%) underwent pylorus-preserving gastrectomy, 44 (15%) underwent proximal gastrectomy, 8 (3%) underwent local resection, and 6 (2%) underwent total gastrectomy. A comparison of the three groups, namely absolute, expanded and surgical criteria, showed significant differences in some factors such as gender, tumor location, circumference, tumor size, pT, surgical procedure, and pathology (Table 1).
SN mapping
The SN detection rate was 99.3% (301/303) and the mean of identified SNs was 4.9 ± 3.0. In the intraoperative frozen section, the metastasis of SN was detected in 5 patients who were all included in surgical criteria, and metastatic SN was identified in 11 patients (3.6%) using formalin-fixed specimens. Single SN basin was identified in 168 (56%) patients, 96 (32%) had 2 SN basins, 33 (11%) had 3 SN basins, and 4 (1%) had 4 SN basins. Figure 1 shows the number of SNs, SN basins, and rate of SN metastasis in the 6 groups.
First, we compared the LNM status among the 3 groups, viz, absolute, expanded and surgical criteria, and found that SN metastasis rate tended to be higher in the surgical criteria group than in the other groups (SN metastasis; p = 0.072, SN metastasis excluding ITC; p = 0.071). Non-SN metastasis, indicating the metastasis to lymph node that is not SN, was seen only in the surgical criteria group (Table 2). Regarding expanded criteria, we compared lymphatic flow, including number of SNs and SN basins, and rate of SN metastasis in 4 groups (group 2–5), and found that patients allocated to group 5 had significantly greater number of SNs and SN basins (Table 3). Although there was no significant difference in SN metastasis, SN metastasis was identified only in group 5. Supplemental Table 1 also shows a comparison of the six groups (groups 1–6).
Moreover, we identified a tumor lesion according to size, location, and circumference in cases with six or more SNs and cases with three or more basins for patients with expanded criteria (Fig. 2 and Supplemental Table 2). In group 5, as well as other groups, the tumor lesion was mainly located on the anterior wall–lesser curvature of the middle stomach.
Tumor lesion based on location and circumference in cases with six or more SNs and cases with three or more basins. The number in gray circle indicates the proportion of lesion circumference; anterior wall, lesser curvature, posterior wall, and greater curvature. The red number indicates the proportion of lesion location; upper third of the stomach, middle third of the stomach, and lower third of the stomach. A–D The tumor lesion of patients in groups 2, 3, 4, and 5, respectively. The upper figure (2A-1, 2B-1, 2C-1, and 2D-1) denotes the tumor lesion in cases with six or more SNs, whereas the lower figure (2A-2, 2B-2, 2C-2, and 2D-2) depicts the tumor lesion in cases with three or more basins
Figure 3 shows details of SN distribution in one patient diagnosed with a 10-mm undifferentiated carcinoma and SN metastasis. In this patient, 8 LNs were identified as SNs and two SN metastasis were defined as isolated tumor cell (ITCs). Notably, metastasis was not evident during the surgical procedure and was diagnosed based on pathological findings. Although single tumor cells were not detected by hematoxylin–eosin stain in the nodal tissue, cytokeratin immunostaining highlights nodal isolated tumor cell cluster with slightly malignant cytologic features.
Details of SN distribution in a patient with SN metastasis. Gray, orange, and yellow colors indicate SN, SN metastasis, and tumor, respectively. a The location of tumor lesion, SN and SN metastasis. b The pathological images (cytokeratin immunostaining) of ITC. Although single tumor cells could not be detected by hematoxylin–eosin staining in the nodal tissue, a high magnification of ITC (blue inset; high-power view) showed that cytokeratin immunostaining highlights the nodal isolated tumor cell cluster with slightly malignant cytologic features
Discussion
We have analyzed the status of lymphatic flow in early gastric cancer based on SN mapping in a cohort of 301 patients and our results show that patients diagnosed with pT1a undifferentiated carcinoma of 20 mm or less can develop a robust lymphatic network and a large lymphatic flow.
Indications for ER in early gastric cancer have been reported, especially in intramucosal undifferentiated adenocarcinoma that is 20 mm or less in size. Hirasawa et al. [19] have reported no LNM in 301 patients with intramucosal cancers that were 20 mm or less in size and without lymphovascular invasion. Furthermore, Yamamoto et al. [20] have suggested the feasibility of ER for undifferentiated adenocarcinoma [20]; therefore, the Japanese multi-institutional Phase II trial (JCOG1009/1010) was conducted to evaluate the efficacy and safety of ER in this patient group [21]. Additionally, as described before, previous studies have reported on the risk of LNM in this patient group. Pessorrusso et al. [22] report 7.7% LNM in patients with intramucosal, undifferentiated adenocarcinoma lesions. Abdelfatah et al. [23] have suggested an expansion of the indications for undifferentiated cancer that are 2 cm or less so that they are balanced with the risk of surgery, given that a meta-analysis has found an increased risk of LNM in these patients. However, in all these studies, the indications for ER have been discussed only based on LNM rate for each lesion. To the best of our knowledge, this is the first report to examine the extent of lymphatic flow based on the SN concept with an aim to reevaluate the expanded criteria. As described by us previously [24, 25], the number of identified SNs may indicate the number of lymphatic flow paths that subsequently cause LNM [26]. Moreover, we believe that lymphatic network development and a large lymphatic flow rate may be present in undifferentiated cancers, even with expanded criteria, and that this may be associated with LNM. Wakisaka et al. [27] have suggested that, in oral squamous cell carcinoma, lymph node lymphangiogenesis occurs prior to metastasis, and that it is associated with VEGF-A and VEGF-D expression. The expression of these proteins may be high in undifferentiated cancer due to higher malignancy rate. Thus, it follows that the mechanism of LNM in SN, which is the initial site of metastasis, can be understood better by verifying lymphangiogenesis.
We only observed ITC, as SN metastasis, in patients diagnosed with intramucosal, undifferentiated adenocarcinoma, measuring 20 mm or less, nonulcerated lesion (group5). The clinical significance of ITC remains unclear; however, Yanagita et al. [28] have clarified the presence of proliferative activity of ITC, determined using IHC (immunohistochemistry) for Ki-67. They have also suggested that ITC should be removed, especially during SN navigation surgery using SN mapping [28]. As we have described in a previous study, it may be sufficient to perform only a SN basin resection when an ITC alone is identified [25]. Taken together, these results imply that the optimal treatment modality in this group of patients may be a combination of ER and SN mapping. However, as the feasibility of SN mapping after ER needs to be established, a multicenter prospective Phase II study has been planned [14]. Another option is to perform non-exposed endoscopic wall inversion surgery (NEWS), a new technique that involves full-thickness partial resection. This technique can minimize the extent of gastric resection during endoscopic and laparoscopic surgery, even without transluminal access [29]. Furthermore, compared to ER, the potential risk of residual cancer and delay in surgical treatment may be avoided.
The results reported here are useful for SN navigation surgery as there are few studies on the association between lymphatic flow and histological type in early gastric cancer. Lavy et al. [30] have reported a higher rate of SLN involvement in a group of patients with poorly differentiated adenocarcinoma than patients with moderately differentiated adenocarcinoma, which is similar to the results from the present study. Additionally, our results suggest that the optimal surgical procedure can be decided based on preoperative histological findings. Last, these results also imply that individualized treatment strategies can be provided based on the patients’ oncological background by combining endoscopic treatment and SN mapping.
Kinami et al. reported that basin distribution depends on the tumor size and location [17]. Thus, we also compared the tumor lesion according to size, location, and circumference in cases with six or more SNs and cases with three or more basins for patients with expanded criteria. The tumor lesions between these groups had no marked difference; therefore, the lymphatic flow may be affected by the affinity of lymphatic invasion rather than the characteristics of the occupying lesion.
The above notwithstanding, this study has some limitations. First, this was a retrospective, single-center study that was limited to Japanese patients. Second, the results of procedures, including SN mapping, can be affected by training status. Third, there were discrepancies in pre- and postoperative histologic diagnosis in tumor lesion. Similarly, Park et al. [31] have reported that 20% of patients diagnosed with differentiated cancer by endoscopic biopsy actually display undifferentiated histology during pathological assessment. They also argue that these results in some tumors may be falsely categorized preoperatively, and that it consequently results in under- or over-estimation of LNM risk. Fourth, because there is no evidence on whether ITC has a negative impact on prognosis, the efficacy of lymph node dissection for ITC remains unclear. Nonetheless, as described before, we suggest that ITC should be resected because of its proliferative activity to provide oncological safety by SN basin excision.
Conclusions
We describe the status of lymphatic flow in early gastric cancer based on SN mapping. Patients with intramucosal, nonulcerated, undifferentiated adenocarcinoma measuring 20 mm or less could develop higher lymphatic networks and larger lymphatic flows than those in patients with adenocarcinoma that satisfy expanded ER criteria. For these patients, careful follow-up is required after endoscopic resection.
References
Kojima T, Parra-Blanco A, Takahashi H, Fujita R. Outcome of endoscopic mucosal resection for early gastric cancer: review of the Japanese literature. Gastrointest Endosc. 1998;48:550–4.
Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer. 2017;20:1–19.
Gotoda T, Yanagisawa A, Sasako M, Ono H, Nakanishi Y, Shimoda T, et al. Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer. 2000;3:219–25.
Kang HJ, Kim DH, Jeon T-Y, Lee SH, Shin N, Chae SH, et al. Lymph node metastasis from intestinal-type early gastric cancer: experience in a single institution and reassessment of the extended criteria for endoscopic submucosal dissection. Gastrointest Endosc. 2010;72:508–15.
Wang H, Zhang H, Wang C, Fang Y, Wang X, Chen W, et al. Expanded endoscopic therapy criteria should be cautiously used in intramucosal gastric cancer. Chin J Cancer Res. 2016;28:348–54.
Oh S-Y, Lee K-G, Suh Y-S, Kim MA, Kong SH, Lee HJ, et al. Lymph node metastasis in mucosal gastric cancer. Ann Surg. 2017;265:137–42.
Wong JH, Cagle LA, Morton DL. Lymphatic drainage of skin to a sentinel lymph node in a feline model. Ann Surg. 1991;214:637–41.
Morton DL, Wen DR, Wong JH, Economou JS, Cagle LA, Storm FK, et al. Technical details of intraoperative lymphatic mapping for early stage melanoma. Arch Surg. 1992;127:392–9.
Giuliano AE, Kirgan DM, Guenther JM, Morton DL. Lymphatic mapping and sentinel lymphadenectomy for breast cancer. Ann Surg. 1994;220:391–8.
Kitagawa Y, Kitano S, Kubota T, Kumai K, Otani Y, Saikawa Y, et al. Minimally invasive surgery for gastric cancer—toward a confluence of two major streams: a review. Gastric Cancer. 2005;8:103–10.
Takeuchi M, Takeuchi H, Kawakubo H, Kitagawa Y. Update on the indications and results of sentinel node mapping in upper GI cancer. Clin Exp Metastasis. 2018;1:1–7.
Takeuchi H, Kitagawa Y. New sentinel node mapping technologies for early gastric cancer. Ann Surg Oncol. 2012;20:522–32.
Kitagawa Y, Takeuchi H, Takagi Y, Natsugoe S, Terashima M, Murakami N, et al. Sentinel node mapping for gastric cancer: a prospective multicenter trial in Japan. J Clin Oncol. 2013;31:3704–10.
Mayanagi S, Takeuchi H, Kamiya S, Niihara M, Nakamura R, Takahashi T, et al. Suitability of sentinel node mapping as an index of metastasis in early gastric cancer following endoscopic resection. Ann Surg Oncol. 2014;21:2987–93.
Sobin L, Gospodarowicz MWC, editors. TNM classification of malignant tumours. 7th ed. New York: Wiley-Liss; 2009.
Sano T, Aiko T. New Japanese classifications and treatment guidelines for gastric cancer: revision concepts and major revised points. Gastric Cancer. 2011;14:97–100.
Kinami S, Fujimura T, Ojima E, Fushida S, Ojima T, Funaki H, et al. PTD classification: proposal for a new classification of gastric cancer location based on physiological lymphatic flow. Int J Clin Oncol. 2008;13:320–9.
Nakahara T, Kitagawa Y, Takeuchi H, Fujii H, Suzuki T, Mukai M, et al. Preoperative lymphoscintigraphy for detection of sentinel lymph node in patients with gastric cancer—initial experience. Ann Surg Oncol. 2008;15:1447–533.
Hirasawa T, Gotoda T, Miyata S, Kato Y, Shimoda T, Taniguchi H, et al. Incidence of lymph node metastasis and the feasibility of endoscopic resection for undifferentiated-type early gastric cancer. Gastric Cancer. 2009;12:148–52.
Yamamoto Y, Fujisaki J, Hirasawa T, Ishiyama A, Yoshimoto K, Ueki N, et al. Therapeutic outcomes of endoscopic submucosal dissection of undifferentiated-type intramucosal gastric cancer without ulceration and preoperatively diagnosed as 20 millimetres or less in diameter. Dig Endosc. 2010;22:112–8.
Takizawa K, Takashima A, Kimura A, Mizusawa J, Hasuike N, Ono H, et al. A phase II clinical trial of endoscopic submucosal dissection for early gastric cancer of undifferentiated type: Japan Clinical Oncology Group Study JCOG1009/1010. Jpn J Clin Oncol. 2012;43:87–91.
Pessorrusso FCS, Felipe-Silva A, Jacob CE, Ramos MF, Ferreira VA, de Mello ES, et al. Risk assessment of lymph node metastases in early gastric adenocarcinoma fulfilling expanded endoscopic resection criteria. Gastrointest Endosc. 2018;88:912–8.
Abdelfatah MM, Barakat M, Lee H, Kim JJ, Uedo N, Grimm I, et al. The incidence of lymph node metastasis in early gastric cancer according to the expanded criteria in comparison with the absolute criteria of the Japanese Gastric Cancer Association: a systematic review of the literature and meta-analysis. Gastrointest Endosc. 2018;87:338–47.
Niihara M, Takeuchi H, Nakahara T, Saikawa Y, Takahashi T, Wada N, et al. Sentinel lymph node mapping for 385 gastric cancer patients. J Surg Res. 2016;200:73–81.
Takeuchi M, Takeuchi H, Kawakubo H, Shimada A, Nakahara T, Mayanagi S, et al. Risk factors for lymph node metastasis in non-sentinel node basins in early gastric cancer: sentinel node concept. Gastric Cancer. 2019;22:223–30.
Kamiya S, Takeuchi H, Nakahara T, Niihara M, Nakamura R, Takahashi T, et al. Auxiliary diagnosis of lymph node metastasis in early gastric cancer using quantitative evaluation of sentinel node radioactivity. Gastric Cancer. 2015;19:1080–7.
Wakisaka N, Hasegawa Y, Yoshimoto S, Miura K, Shiotani A, Yokoyama J, et al. Primary tumor-secreted lymphangiogenic factors induce pre-metastatic lymphvascular niche formation at sentinel lymph nodes in oral squamous cell carcinoma. PLoS O ne. 2015;10:e0144056.
Yanagita S, Natsugoe S, Uenosono Y, Kozono T, Ehi K, Arigami T. Sentinel node micrometastases have high proliferative potential in gastric cancer. J Surg Res. 2008;145:238–43.
Takeuchi H, Goto O, Yahagi N, Kitagawa Y. Function-preserving gastrectomy based on the sentinel node concept in early gastric cancer. Gastric Cancer. 2017;20:53–9.
Lavy R, Kapiev A, Hershkovitz Y, Poluksht N, Rabin I, Chikman B, et al. Tumor differentiation as related to sentinel lymph node status in gastric cancer. World J Gastrointest Surg. 2014;27(6):1–4.
Park JY, Ryu KW, Eom BW, Yoon HM, Kim SJ, Cho SJ, et al. Proposal of the surgical options for primary tumor control during sentinel node navigation surgery based on the discrepancy between preoperative and postoperative early gastric cancer diagnoses. Ann Surg Oncol. 2013;21:1123–9.
Acknowledgements
The authors thank Kumiko Motooka, who is a staff member at the Department of Surgery at the Keio University School of Medicine, for her help in the preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Yuko Kitagawa received Grant support from Taiho Pharmaceutical Co., Ltd, Chugai Pharmaceutical Co., Ltd., Yakult Honsha Co. Ltd., Daiichi Sankyo Company, Limited, Merck Serono Co., Ltd., Asahi Kasei Co., Ltd., EA Pharma Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., Otsuka Pharmaceutical Factory Inc., Shionogi & Co., Ltd., Kaken Pharmaceutical Co., Ltd., Kowa Pharmaceutical Co., Ltd., Astellas Pharma Inc., Medicon Inc., Dainippon Sumitomo Pharma Co., Ltd., Taisho Toyama Pharmaceutical Co., Ltd., Kyouwa Hakkou Kirin Co., Ltd., Pfizer Japan Inc., Ono Pharmaceutical Co., Ltd., Nihon Pharmaceutical Co., Ltd., Japan Blood Products Organization, Medtronic Japan Co., Ltd., Sanofi K.K., Grants from Eisai Co., Ltd., Tsumura & Co., KCI Licensing, Inc., Abbott Japan Co., Ltd., and Fujifilm Toyama Chemical Co., Ltd.
Ethical standards
All procedures were followed in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions. Informed consent or substitute for it was obtained from all patients for being included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Takeuchi, M., Kawakubo, H., Shimada, A. et al. Assessment of lymphatic flow based on the sentinel node concept in early gastric adenocarcinoma that satisfies expanded endoscopic resection criteria. Gastric Cancer 23, 531–539 (2020). https://doi.org/10.1007/s10120-019-01026-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10120-019-01026-7