Abstract
Background
Several efforts have been made to alleviate harms and symptoms after gastrectomy for gastric cancer. We previously conducted a randomized controlled trial (CCOG1101) to compare quality of life (QOL) and nutritional status between Roux-en-Y (RY) and aboral pouch (AP) reconstructions for up to 1 year after total gastrectomy. However, long-term outcomes after AP reconstruction remain unclear.
Methods
A prospective multicenter observational study was conducted to compare QOL, body composition, and nutritional indicators between the RY and AP reconstructions at 5 years after surgery among patients who were enrolled in the CCOG1101 trial. QOL was assessed by the PGSAS-37 questionnaires as well as the EORTC QLQ-C30 and STO22.
Results
Sixty patients (31 for RY and 29 for AP) were recruited for analysis. There were no significant differences in baseline and perioperative characteristics between the two groups. No significant differences were found in the EORTC QLQ-C30 global health status and functional scales. Regarding symptom scales in the QLQ-C30 and STO22, a more favorable score for the diarrhea scale was observed in the AP group. Diarrhea was also the only item in the PGSAS-37 questionnaires in which significant benefit of AP was observed. Body weight and lean body mass continued to decrease throughout the postoperative 5 years in both groups. None of the conventional nutritional indicators using the serum samples showed significant difference between the two groups.
Conclusions
Long-term observation suggested little benefit of AP reconstruction after total gastrectomy other than in alleviating diarrhea.
Similar content being viewed by others
Introduction
Almost one million new cases of gastric cancer (GC) were estimated to have occurred in 2012, making it the fifth most common malignancy in the world [1]. Surgical resection is the mainstay of treatment for patients with GC and total gastrectomy is mandatory to achieve curative resection of tumors invading the upper part of the stomach. Roux-en-Y (RY) has long been the commonest type of reconstruction after total gastrectomy worldwide [2]. However, the loss of reservoir capacity is one of the main reasons for post-gastrectomy symptoms that lead to the deteriorated quality of life (QOL) and nutritional status [3,4,5], and RY in theory does not overcome this deficit.
Jejunal pouch has been proposed as a method to compensate for the loss of reservoir capacity and reportedly reduced the incidence of early and late dumping symptoms leading to increase of the serum total protein [6, 7]. However, pouch reconstruction is more expensive and is sometimes associated with food stasis and excessive pouch dilatation [8]. More recently, aboral pouch (AP) reconstruction, in which a jejunal pouch is created in the Y limb of the RY reconstruction, has attracted attention due to its simplicity. In the original report by Horvath [9] that compared AP with RY, significantly higher serum cholesterol levels and superior postoperative quality of life in terms of the Gastrointestinal Quality of Life Index (GIQL1) score were observed in the AP group. Since this study was a single-center comparison analyzing short-term QOL with no data on body composition, we conducted a prospective multicenter randomized trial to compare nutritional status and QOL assessed by the European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire-Core 30 (QLQ-C30) and Stomach Module (STO22) [10] between the two groups (CCOG1101 / UMIN - CTR 000006155) [11], but failed to show benefit of AP over RY within 12 months postoperatively. We hypothesized, however, that the reconstruction procedure could still influence the QOL and nutritional outcomes in the long term [12]. Moreover, Postgastrectomy Syndrome Assessment Scale (PGSAS) [13], new integrated questionnaires to more specifically address the issue of QOL after gastrectomy, have recently emerged and shown promise in several observational studies [14,15,16].
To explore the long-term outcomes after AP reconstruction, we conducted a prospective multicenter observational study to compare QOL and nutritional status between the RY and AP reconstruction at 5 years after surgery among patients who were enrolled in the CCOG1101 trial, using the PGSAS-37 in addition to the EORTC QLQ-C30 and STO22.
Materials and methods
This prospective observational study was approved by an internal review board at 10 participating institutions after review of the scientific and ethical validity of the protocol and was registered in the University Hospital Medical Information Network (UMIN) Clinical Trial Registry as UMIN000037443 (http://www.umin.ac.jp/ctr/index.htm).
Patient eligibility
Patients who were registered and analyzed in the CCOG1101 study, and from whom written informed consent was obtained for this observational study were eligible. Patients who had apparent recurrent disease were ineligible. Patients who were unable to complete the questionnaires due to advanced age or other reasons were excluded. Briefly, eligibility criteria of the CCOG 1101 trial included patients aged 20–80 years who underwent total gastrectomy for histologically confirmed adenocarcinoma of the stomach. Patients with cancer of the gastric remnant, those who failed to undergo R0 resection, those who were under systemic administration of corticosteroids and those with uncontrolled diabetes mellitus or other severe systematic diseases were excluded [11].The schemas of the RY and AP reconstruction were depicted in Supplemental Fig. 1. Although patients with other malignancies were excluded in the CCOG1101 trial, it was not prescribed in a study protocol of the current study.
Study parameters
The patients were asked to fill in the EORTC QLQ-C30, STO22, and PGSAS-37 questionnaires at 5 years after surgery. Body weight, body fat mass, and lean body mass were measured using the Body Composition Analyzer DC-320 (TANITA, Tokyo, Japan). Serum total protein, albumin, total cholesterol, triglyceride, calcium, iron, total lymphocyte count, and hemoglobin were measured as nutritional indicators. All data of body composition and blood testing were collected at 5 years after surgery.
Assessment of postoperative QOL
The EORTC QLQ-C30 is composed of the global health status, 5 functional scales (physical, role, cognitive, emotional, and social), and 9 symptom scales (fatigue, pain, nausea and vomiting, dyspnea, sleep disturbance, appetite loss, constipation, diarrhea, and financial difficulties) [10, 14]. The STO22 is an additional questionnaire specific for GC patients that is composed of 22 items [17,18,19]. The items are categorized into 5 symptom scales (dysphagia, abdominal pain, reflux, eating restriction, and anxiety) and 4 single items (dry mouth, taste problems, body image, and hair loss). Missing data were processed according to the EORTC scoring manuals [20]. All scales were transformed to scores of 0 to 100 [10]. The PGSAS-37 is a newly developed set of integrated questionnaires specifically designed for assessment of postoperative symptoms and QOL after gastrectomy. Main outcome measures of the PGSAS-37 are composed of 7 symptom scales (Esophageal reflux, abdominal pain, meal-related distress, indigestion, diarrhea, constipation, and dumping), 4 living status scales (ingested amount of food per meal, necessity for additional food, quality of ingestion, and ability for working), and 1 QOL scale (dissatisfaction for daily life). Total symptom score is calculated by the average of 7 symptom scales. In the EORTC questionnaire, high scores denoted favorable outcomes regarding global health status and functioning scales, whereas low scores on the symptom scales and items indicated favorable outcomes. In the PGSAS-37 questionnaire, high scores denoted favorable outcomes regarding ingested amount of food per meal and quality of ingestion, whereas low scores on the symptom scales, necessity for additional food, ability for working, and dissatisfaction for daily life indicated favorable outcomes. The questionnaire sheets were sent directly from the data center to the patient at 5 years after surgery and returned to the data center after completion by the patients.
Endpoints and data integration
The primary endpoint was comparison of the QOL 5 years after total gastrectomy between the RY and AP reconstruction groups. The secondary endpoints were changes in body weight, body fat mass, lean body mass, and levels of nutritional indicators measured by the blood tests. Data on the EORTC QLQ-C30, STO22, body composition, and nutritional indicators before surgery (baseline), and at postoperative year (POY) 1 were retrieved from case report forms of the CCOG1101 trial.
Statistical analysis
Comparisons of the continuous variables were made using the t-test. The Fisher’s exact test was used to compare categorical variables. No statistical adjustments for multiplicity were conducted because of a hypothesis exploratory analysis. P value < 0.05 was considered to be statistically significant. In the case of P < 0.1, Cohen’s d was calculated. Interpretation of effect sizes were 0.2 < small, 0.5 < medium, and 0.8 < large in Cohen’s d. Statistical analysis of the data was performed using a JMP software program (version 13, SAS Institute, Inc, Cary, NC).
Results
Patients and perioperative factors
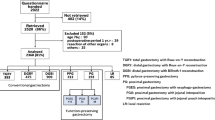
As shown in the flowchart, 83 patients (41 for the RY group and 42 for the AP) underwent QOL analysis at 12 months after surgery in the CCOG1101 study (Fig. 1). In the RY group, 4 patients died from other diseases, 3 patients were lost to follow-up, 2 patients had a recurrence of gastric cancer, and a physician in charge failed to ask the patient for participation in one patient. In the AP group, 4 patients died from other diseases, 3 patients were deemed to be ineligible by physicians due to cognitive impairment, 2 patients refused to be enrolled, one patient was lost to follow-up, one patient had a recurrence of gastric cancer, and the physicians in charge failed to ask the patient for participation in 2 patients. Eventually, written informed consent to participate in the current study was obtained from 60 patients, and these patients were eligible for the current data analysis between January 2016 and November 2017. The completion rates of the questionnaires were 90.3% and 100% in the RY and AP group, respectively (Supplemental Table 1).
Patient baseline characteristics and perioperative data were summarized in Table 1. There were no significant differences in gender, preoperative body weight, preoperative body mass index, extent of lymphadenectomy, splenectomy, postoperative complication rates, pathological stage, and adjuvant chemotherapy between the two groups. The patients in the AP group tended to be older. There were two patients who underwent surgery for cancer (lung cancer in the RY group and esophageal cancer in the AP group) during the surveillance period. In the RY group, seven patients underwent postoperative adjuvant S-1 monotherapy. In the AP group, postoperative adjuvant S-1 monotherapy was performed in three patients and capecitabine plus oxaliplatin was performed in two patients. Thus, the proportion of patients who underwent chemotherapy was comparable between the groups.
EORTC QLQ-C30
When compared with POY1, no improvement at POY5 was observed for either groups regarding the scores for the EORTC QLQ-C30 global health status and physical, role, and social functioning (Fig. 2). The scores for emotional functioning were gradually improved over baseline level at 5 years postoperatively in both groups. The scores for cognitive functioning declined at POY 5 compared to POY 1 in both groups. There were no significant differences between the two groups in any of these parameters at POY 5.
As for the QLQ-C30 symptoms scale, the diarrhea score, as indicated by % change from baseline, showed significant improvement in the AP group compared to RY group at POY 5 (6.90 ± 20.66 and 21.43 ± 30.83, respectively; P = 0.032). The scores for sleep disturbance, appetite loss, and constipation were improved at POY 5 compared to POY 1 only in the AP group (Fig. 3).
EORTC STO22 symptom scales
The chronological changes in scores of the EORTC STO22 symptom scales are shown in Fig. 4 The AP group tended to have better scores for abdominal pain and taste problem, but the differences did not reach statistical significance (Fig. 4).
PGSAS-37 symptom scores
PGSAS-37 symptom scores at POY 5 were listed in Table 2. Consistent with results in the EORTC QLQ-C30, the diarrhea score of PGSAS-37 was significantly lower in the AP group compared to the RY group (1.93 ± 0.87 and 2.60 ± 1.49, respectively; P = 0.044). Cohen’s d of diarrhea score between two groups was 0.55 and the effect size was intermediate.
Changes in body compositions and nutritional indicators
The body weight, body fat mass, and lean body mass were equivalently decreased at POY 1 both in the RY and AP groups. Overall, the levels of body weight and lean body mass continued to decrease from POY 1 to POY 5, while the body fat mass was observed to recover marginally at POY 5 (Table 3).
With respect to nutritional indicators determined by blood tests, there were no significant differences between the RY and AP reconstruction groups. Most of the indicators, with the exception of total lymphocyte count and serum iron, remained below the baseline levels at POY 5 both in the RY and AP groups (Table 3).
Discussion
Although various reconstruction procedures have been proposed and evaluated, identification of the optimal mode of reconstruction following total gastrectomy remains an unsolved task. Reconstruction with a jejunal pouch after total gastrectomy reportedly confers patients with enhanced food intake and decreased symptoms related to dumping syndrome and reflux, resulting in improvements in the QOL [12, 21]. Nevertheless, several surgeons avoid creating a jejunal pouch because of complexity in surgical procedure and expense (an additional surgical stapler costs approximately $300, in Japan). Furthermore, we were unable to prove benefit of a pouch procedure in our previous randomized trial. We nevertheless conducted a prospective multicenter observational study to look at QOL and nutritional status 5 years after surgery with the patients who had participated in our previous trial, with a hope that some benefits may be found with the pouch reconstruction in the long term. However, no differences in the body composition and nutritional status in favor of AP were observed, and superiority of the AP reconstruction was denied from these viewpoints. Another important finding in this additional follow-up was that the body composition and nutritional indicators did not recover to the baseline levels even at POY 5 regardless of the reconstruction methods.
Of note, improvement in the diarrhea scale between POY 1 and 5 was observed exclusively in the AP group both through measurement of the EORTC QLQ-C30 symptoms scale and the PGSAS-37 score. In addition, scores for sleep disturbance showed a similar trend and led us to speculate that decreased frequency of diarrhea relieved patients of sleep disturbance. Insertion of the jejunal pouch with irregular or suppressed motility pattern as well as the enlarged reservoir capacity could have contributed to prevent diarrhea by slowing down the food passage through the small intestines [9]. Further studies are warranted to uncover the influence of the AP on digestive functions through analyses of lipid absorption and stool fat content in a larger cohort. Nevertheless, diarrhea was the only symptom scale in which the AP group demonstrated a superior outcome. Since the AP procedure confers a definite disadvantage in terms of cost for an additional surgical stapler, RY should remain a standard reconstruction method after total gastrectomy.
In the original study reported by Harvest et al. a jejuno-jejunostomy in the RY reconstruction was completed by a simple side-to-end hand-sewn anastomosis [9]. On the other hand, a jejuno-jejunostomy of the RY group in the current study was created by side-to-side anastomosis using a 75-mm linear stapler, and that actually resulted in creation of a small pouch-like structure. This may have marred the effect conferred by the 12-cm pouch created in the AP group and could have been a reason that we failed to detect the differences in favor of the AP group in the current study.
One potentially serious adverse event caused by the pouch is delayed emptying of the pouch, which gives a sensation of epigastric fullness or nausea and leads to poor food intake [23, 24]. Chronic accumulation of a large amount of food residue can result in dysfunctional dilatation of the pouch. Ikeda et al. even postulated that the pouch should be made smaller than what is actually intended, taking into consideration its future dilatation, and disclosed their recommendation of the optimal length of jejunal pouch to be placed between the esophagus and duodenum [25]. In the current study, the Y limb in the AP group was to be created into a 12-cm pouch by side-to-side anastomosis using two 75-mm linear staplers. We consider size of the jejunal pouch to have been appropriate, since there was no case with serious complaints in this regard among patients who were recruited for this study.Ongoing postoperative care, such as nutritional support and daily life guidance, is advisable over the long period for patients who underwent total gastrectomy.
The current study has several limitations. First, the sample size was relatively small and was not calculated based on any hypothesis. This was attributable to the fact that the study had not been preplanned and the data were derived from extended observation of patients who were recruited in a prospective randomized trial. Thus, some patients were excluded from the original cohort due to lack of either the will or ability to participate in this study, sometimes because of aging. Second, the PGSAS-37 scores at baseline and 1POY were unavailable since this instrument had not been used in the previous randomized study. Third, a larger sample size is required to perform statistical adjustments for multiplicity, which has not been carried out in the current study. Last, since absence or presence of other malignancies was not prescribed in the a study protocol of the current study (CCOG1505), two patients (3.3%) who underwent surgery for cancer diagnosed during the surveillance period were included in the analysis.
In summary, we conducted 5-year observation to compare long-term QOL and nutritional status between the RY and AP reconstruction and found little benefit in the AP group with the exception that the diarrhea scores were favorable both in terms of EORTC QLQ-C30 symptoms scale and PGSAS-37 questionnaires.
References
GLOBOCAN. 2012. http://globocan.iarc.fr/old/FactSheets/cancers/stomach-new.asp.
Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer. 2017;20:1–19.
Katsube T, Konnno S, Murayama M, Kuhara K, Sagawa M, Yoshimatsu K, et al. Changes of nutritional status after distal gastrectomy in patients with gastric cancer. Hepatogastroenterology. 2008;55:1864–7.
Kiyama T, Mizutani T, Okuda T, Fujita I, Tokunaga A, Tajiri T, et al. Postoperative changes in body composition after gastrectomy. J Gastrointest Surg. 2005;9:313–9.
Armbrecht U, Lundell L, Lindstedt G, Stockbruegger RW. Causes of malabsorption after total gastrectomy with Roux-en-Y reconstruction. Acta Chir Scand. 1988;154:37–41.
Lygidakis NJ. Long term results of a new method of reconstruction for continuity of the alimentary tract after total gastrectomy. Surg Gynecol Obstet. 1984;158:335–8.
Nakane Y, Okumura S, Akehira K, Okamura S, Boku T, Okusa T, et al. Jejunal pouch reconstruction after total gastrectomy for cancer. A randomized controlled trial. Ann Surg. 1995;222:27–35.
Tono C, Terashima M, Takagane A, Abe K. Ideal reconstruction after total gastrectomy by the interposition of a jejunal pouch considered by emptying time. World J Surg. 2003;27:1113–8.
Horvath OP, Kalmar K, Cseke L, Poto L, Zambo K. Nutritional and life-quality consequences of aboral pouch construction after total gastrectomy: a randomized, controlled study. Eur J Surg Oncol. 2001;27:558–63.
Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–76.
Ito Y, Yoshikawa T, Fujiwara M, Kojima H, Matsui T, Mochizuki Y, et al. Quality of life and nutritional consequences after aboral pouch reconstruction following total gastrectomy for gastric cancer: randomized controlled trial CCG1101. Gastric Cancer. 2016;19:977–85.
Fein M, Fuchs KH, Thalheimer A, Freys SM, Heimbucher J, Thiede A. Long-term benefits of Roux-en-Y pouch reconstruction after total gastrectomy: a randomized trial. Ann Surg. 2008;247:759–65.
Nakada K, Ikeda M, Takahashi M, Kinami S, Yoshida M, Uesono Y, et al. Characteristics and clinical relevance of postgastrectomy syndrome assessment scale (PGSAS)-45: newly developed integrated questionnaires for assessment of living status and quality of life in postgastrectomy patients. Gastric Cancer. 2015;18:147–58.
Takiguchi N, Takahashi M, Ikeda M, Inagawa S, Ueda S, Nobuoka T, et al. Long-term quality-of -life comparison of total gastrectomy and proximal gastrectomy by postgastrectomy syndrome assessment scale (PGSAS-45): a nationwide multi-institutional study. Gastric Cancer. 2015;18:407–16.
Fujita J, Takahashi M, Urushihawa T, Tanabe K, Kodera Y, Yumiba T, et al. Assessment of postoperative quality of life following pylorus-preserving gastrectomy and Billroth-I distal gastrectomy in gastric cancer patients: results of the nationwide postgastrectomy syndrome assessment study. Gastric Cancer. 2016;19:302–11.
Takahashi M, Terashima M, Kawahira H, Nagai E, Uenosono Y, Kinami S, et al. Quality of life after total vs distal gastrectomy with Roux-en-Y reconstruction: use of the postgastrectomy syndrome assessment scale-45. World J Gastroenterol. 2017;23:2068–76.
Kobayashi K, Takeda F, Teramukai S, Gotoh I, Sakai H, Yoneda S, et al. A cross-validation of the European Organization for Research and Treatment of Cancer QLQ-C30 (EORTC QLQC30) for Japanese with lung cancer. Eur J Cancer. 1998;34:810–5.
Blazeby JM, Conroy T, Bottomley A, Vickery C, Arraras J, Sezer O, et al. Clinical and psychometric validation of a questionnaire module, the EORTC QLQ-STO 22, to assess quality of life in patients with gastric cancer. Eur J Cancer. 2004;40:2260–8.
Morita S, Kaptein AA, Oba K, Sakamoto J. The domain structure of the EORTC QLQ-STO22 supported by Japanese validation data. Psychooncology. 2008;17:474–9.
Fayers PM, Aaronson NK, Bjordal K. EORTC QLQ-C30 scoring manual. 3rd ed. Brussels: EORTC; 2001. pp. 10–2.
Gertler R, Rosenberg R, Feith M, Schuster T, Friess H. Pouch vs. no pouch following total gastrectomy: meta-analysis and systematic review. Am J Gastroenterol. 2009;104:2838–51.
Yu W, Park KB, Chung HY, Kwon OK, Lee SS. Chronological changes of quality of life in long-term survivors after gastrectomy for gastric cancer. Cancer Res Treat. 2016;48:1030–6.
Tamura T, Inagawa S, Terashima H, Akashi Y, Hisakura K, Enomoto T, et al. A long-term follow-up result of pouch plasty for severe dysfunction of jejunal pouch reconstruction after total gastrectomy: a case report. Int Surg. 2015;100:954–7.
Katsube T, Konno S, Hamaguchi K, Shimakawa T, Naritaka Y, Ogawa K. Complications after proximal gastrectomy with jejunal pouch interposition: report of a case. Eur J Surg Oncol. 2005;31:1036–8.
Ikeda M, Ueda T, Shiba T. Reconstruction after total gastrectomy by the interposition of a double jejunal pouch using a double stapling technique. Br J Surg. 1998;85:398–402.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Kodera reports grants and personal fees from Johnson & Johnson, Covidien, and Otsuka Pharmaceutical Factory. Other authors declare that there is no conflict interest.
Ethical standards
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions. Informed consent to be included in the study, or the equivalent, was obtained from all patients.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10120_2018_893_MOESM1_ESM.tif
Supplementary material 1: Supplemental Fig. 1: The Y limb in aboral pouch group was created side-to-side anastomosis using linear staplers. (TIF 273 KB)
Rights and permissions
About this article
Cite this article
Tanaka, C., Kanda, M., Murotani, K. et al. Long-term quality of life and nutrition status of the aboral pouch reconstruction after total gastrectomy for gastric cancer: a prospective multicenter observational study (CCOG1505). Gastric Cancer 22, 607–616 (2019). https://doi.org/10.1007/s10120-018-0893-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10120-018-0893-z