Abstract
Background
HER2 and topoisomerase 2 alpha (TOP2A) genomic status was previously reported to predict benefit from anthracyclines in breast cancer. We sought to define the prognostic impact and possible pitfalls related to these biomarkers in resectable gastroesophageal adenocarcinoma.
Methods
HER2 and TOP2A gene amplification by fluorescent in situ hybridization and HER2 protein expression by immunohistochemistry (IHC) were assessed on whole tissue sections from 101 patients receiving peri- or postoperative epirubicin-based chemotherapy. In a subgroup of patients, at least two matched tumor blocks, originating either from surgical procedures (n = 88) or diagnostic biopsies (n = 32), were available for HER2 analyses by IHC.
Results
Eighteen of 101 patients (17.8 %) were HER2 positive, whereas TOP2A was amplified in 4 of 84 patients (4.7 %). HER2 positivity was significantly associated with improved disease-free survival [HR = 0.47 (95 % CI 0.22–0.99), P = 0.046] and overall survival [HR = 0.33 (95 % CI 0.13–0.83), P < 0.018], independent of clinical-pathologic features. HER2 expression in matched tumor blocks from the same resection specimen was discordant in up to 11.8 % of pairs, while this rate increased up to 27.2 % when diagnostic biopsies and paired surgical samples were compared.
Conclusions
HER2 status is an independent prognostic biomarker in gastroesophageal adenocarcinomas receiving epirubicin-based chemotherapy. Compared to diagnostic biopsies, HER2 assessment in multiple resection specimens might lower the risk of sampling errors. These findings have several implications with respect to the optimal choice of the sample to be submitted to IHC testing of HER2.
Similar content being viewed by others
Introduction
Radical surgical resection of the primary tumor and the lymph node basin is the keystone of curative treatment in gastroesophageal adenocarcinoma. However, because between 25 and 50 % of patients experience recurrence after surgery, additional modalities, including systemic treatments and radiotherapy, have been explored in order to improve survival outcomes. In particular, a perioperative chemotherapy regimen containing an anthracycline (epirubicin), cisplatin, and infused 5-fluorouracil (5-FU), commonly referred to as the ECF regimen, has been shown to significantly increase the 5-year overall survival (OS) in comparison with surgery alone [1]. Whereas alternative non-anthracycline regimens have been successfully evaluated [2], a perioperative approach with ECF is still a standard of care in most European countries for treatment of resectable disease. Additionally, despite the lack of a global standard [3], use of anthracyclines is supported by a meta-analysis highlighting significant survival advantages with anthracycline-containing triplets in a palliative setting [4]. As a result, regimens such as ECF (or ECF modifications) are recommended by current European and North American guidelines for both resectable and first-line settings [5–7].
Although it may appear rather counterintuitive, postoperative regimens including epirubicin, cisplatin, and 5-FU, alone or in combination with radiotherapy, failed to prove superior over 5-FU alone [8, 9]. In fact, reliable biomarkers guiding the management of patients with resectable gastroesophageal adenocarcinomas are still lacking.
In breast cancer, despite conflicting results, observational studies indicated that HER2 amplification or HER2 overexpression, along with topoisomerase 2 alpha (TOP2A) genomic abnormalities, is a potential predictive biomarker of benefit from adjuvant epirubicin-based chemotherapy [10]. The topoisomerase 2 alpha protein is the gene product of TOP2A, and it is a key molecular target of anthracyclines. Located at chromosome band 17q12-q21, the HER2 gene is adjacent to TOP2A, and both genes can be simultaneously co-amplified in primary breast tumors and breast cancer cell lines [11]. These observations raised the possibility of a functional link among HER2 positivity, TOP2A aberrations, and responsiveness to topoisomerase 2 alpha inhibitors commonly employed in the adjuvant treatment.
Unlike breast cancer, the value of HER2 positivity in gastroesophageal adenocarcinomas as a predictive marker for anthracycline sensitivity has never been demonstrated, while its prognostic value remains unclear with inconsistent findings across different series [12]. On the other hand, assessment of HER2 expression by immunohistochemistry (IHC) is possibly challenged by a substantial heterogeneity of HER2 staining [13–16], which carries an inherent risk of sampling errors.
In gastroesophageal carcinomas HER2 amplification rates range between 10 and 20 %, while TOP2A was earlier reported as being co-amplified in the majority of HER2-amplified cancers, with frequencies even higher than in breast cancer [17]. However, despite a broad use of anthracyclines in this context, to the best of our knowledge, the value of TOP2A alterations to predict efficacy of the ECF regimen has never been explored. Given these premises, we therefore sought to determine whether HER2 and/or TOP2A status would constitute prognostic markers suggesting survival benefits from epirubicin-based chemotherapy in patients with resectable gastroesophageal adenocarcinoma.
Materials and methods
This study includes 101 patients with localized gastric or gastroesophageal junction adenocarcinomas who were surgically treated with curative intent between 2000 to 2012 at Humanitas Research Hospital. Surgical procedures consisted of either total or subtotal gastrectomy with a D2 lymph node resection and distal esophageal resection, where indicated. In addition, all patients were treated with either postoperative (68.4 %) or perioperative (31.6 %) chemotherapy: ECF [(epirubicin (50 mg/m2), cisplatin (60 mg/m2), and continuous infusional 5-FU (200 mg/m2 per day)] in 86 patients (85.2 %) or adjuvant weekly PELF [cisplatin (40 mg/m2), 6S-leucovorin (250 mg/m2), epirubicin (35 mg/m2), 5-FU (500 mg/m2), and glutathione (1.5 g/m2)] in 15 patients (14.8 %), respectively. The latter regimen was delivered in the frame of a randomized controlled trial run by the Italian Group for the Study of Digestive Tract Cancer [8]. Follow-up from the end of chemotherapy was performed at 3-month intervals for 2 years, then at 6-month intervals for 3 years, and then yearly. The study approval was obtained by the Institutional Review Board according to the national requirements. All tumors were classified according to the Laurén classification, and their TNM stage was reviewed on the basis of the seventh edition of the UICC guidelines [18] by one gastrointestinal pathologist who was blinded to outcome. In those patients receiving perioperative chemotherapy, histopathological tumor regression was evaluated according to Becker et al. [19].
Immunohistochemical analyses
For each patient, IHC was primarily carried out on whole tissue sections from one or more tumor-containing representative blocks originating from a resection specimen. Also, whenever possible, additional IHC analyses were carried out on matched pretreatment diagnostic biopsies. HER2 expression was detected by applying a commercial immunostaining protocol, according to the manufacturer’s instructions [PATHWAY HER-2/neu (4B5) rabbit monoclonal antibody (Ventana Medical Systems, Milan, Italy] using the automated Benchmark XT platform (Ventana Medical Systems). Staining intensity was evaluated using the 0–3+ scale according to the modified IHC scoring system adopted in the ToGA study [20]. At least five positive cancer cells within a cluster of a tissue biopsy sample or at least 10 % of neoplastic cells positive in a surgical specimen are necessary for this scoring system. Discrepant results were defined in the event of low HER2 expression levels (score 0/1+) in one sample that turned high (2+/3+) in at least one other matched sample, independent from the origin of the sample (diagnostic biopsy vs. surgical specimen). The criteria for such dichotomization of HER2 expression levels were based on a previously published post hoc analysis of ToGA [20].
Fluorescence in situ hybridization (FISH): HER2 and TOP2A
Only equivocal HER2 scores (2+) by IHC in either the biopsy or resection specimens were further characterized by FISH on a sequential serial section. Tissue was processed using the PathVysion HER2 DNA Probe Kit (Abbott Molecular) according to the manufacturer’s protocol. The HER2 gene was considered amplified if the HER2-to-chomosome 17 centromere (CEP17) ratio was ≥2.0 and if the average HER2 gene copy number was at least 6 [21]. Regardless of HER2 status, TOP2A genomic alterations were measured in at least one whole tissue section per patient, next to the section where HER2 was investigated. The probes to measure TOP2A alterations were the Locus Specific Identifier TOP2A Spectrum Orange and the CEP17 (D17Z1) Spectrum Green probe (both from Abbott Molecular), which is specific for the centromeric region of chromosome 17. Tumors were considered to have amplified TOP2A if the TOP2A-to-CEP17 ratio was ≥2.1, to have deleted TOP2A if the ratio was 0.8:1 or less, and to have normal TOP2A if the ratio was between 0.8:1 and 2:1 [22]. Both TOP2A and HER2 gene copy numbers were determined in a minimum of 20 interphase, nonoverlapping tumor cell nuclei and compared with CEP17 to normalize gene copies according to chromosome numbers in those same nuclei.
Definition of HER2-positive status
For both resection specimens and diagnostic biopsies, HER2-positive status was defined by strong (3+) or moderate (2+) immunostaining, with HER2-positive FISH results [in agreement with the European Medicines Agency (EMA) definition for trastuzumab eligibility].
Statistical analysis
Data were summarized as frequencies and proportion or as median and range. Differences between groups were test by the chi-square test or the Fisher exact test when appropriated. OS was measured from the date of surgery until death from any cause, with observations censored for patients last known alive. Disease-free survival (DFS) was measured from surgery until documentation of progressive disease or death from any cause, with observations censored for patients last known alive without report of relapse. Distributions of OS and DFS were estimated by the method of Kaplan and Meier. The Cox proportional hazard model was used to estimate the hazard ratios (HRs) with their corresponding 95 % confidence intervals (95 % CI). All P values are two-tailed. All analyses were performed using STATA software v. 13. Analyses were based on the data available at last follow-up in 2014.
Results
Patients characteristics
A total of 101 patients were enrolled in the study. Baseline characteristics have been described in detail and are summarized in Table 1.
With a median follow-up of 84.7 months, 62 patients (61.4 %) had a recurrence or death, corresponding to a 5-year DFS of 40.9 % (median 24.6 months), whereas the 5-year overall survival was 40.9 % (median 76.5 months). T-category (P = 0.008), N-category (P < 0.001), timing of chemotherapy (P < 0.001), and primary tumor location (P = 0.023) were significantly associated with DFS in univariate analysis. Similar associations were also observed with respect to OS (Table 1). Timing of chemotherapy and primary tumor location were significantly associated (P < 0.001), thus justifying the use of a composite variable to be entered into the subsequent multivariable model. On the other hand, timing of chemotherapy was not associated with other prognostically relevant clinicopathological characteristics (Table S1).
HER2 status in paired samples and correlation with clinicopathological factors
In the diagnostic algorithm for HER2 assessment, IHC analysis represents the first step to be undertaken. In 88 patients, two blocks obtained from the same resection specimen were retrieved, and for 76 of them, a third additional block was also available. Per patient, the median number of biopsy fragments retrieved was five (range 1–8).
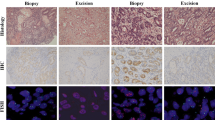
Despite an overall agreement in the rates of 0/1+ and 2+/3+ scores among the three sets of paired surgical specimens (whole tissue sections), when comparing set 1 and set 2, HER2 expression levels were discrepant in 10.2 % of cases (score 0/1+ on one sample that turned 2+/3+ on the other one). Similar figures were observed in an additional comparison involving surgical set 1 and set 3 (Table 2). Higher rates of discrepancies (score 2+/3+ on the biopsy that turned 0/1+ on the surgical specimen and vice versa) were observed comparing one biopsy specimen (obtained pretreatment) with one or more matched surgical samples (Fig. 1). In particular, HER2 expression levels were discrepant in 24.2, 25, and 31 % of cases, when comparing the diagnostic biopsy sets with surgical sets 1, 2, and 3, respectively. Across the three different sets of surgical samples, no significant differences in terms of HER2 expression (scores 2+/3+ vs. 0/1+) were detected according to the timing of chemotherapy (P = 0.477), although the number of samples included in this subset analysis was low (Table 3).
When HER2 status was categorized as negative/positive according to the composite EMA definition, discrepancies of HER2 expression between diagnostic biopsy and matched surgical samples were detected in 5 out of 32 pairs (15.6 %) analyzed (Table 4).
Considering the whole cohort of 101 patients, 74 (73 %) showed a score of 0/1+, 14 (14 %) a score of 2+, and 13 (13 %) a score of 3+. Overall, when the highest IHC score obtained per individual patient was 2+, further FISH analysis revealed HER2 amplification in five additional cases. In all, the rate of HER2 positivity (according to the EMA definition) was 17.8 % (18 of 101). Twelve of 18 HER2-positive patients had intestinal-type histology, but this association was not statistically significant. Moreover, there was no significant association with sex, age, anatomical site, tumor stage, nodal involvement, systemic treatment, and its timing (perioperative vs. postoperative).
TOP2A alterations and correlations with outcome
TOP2A genomic aberrations were analyzed in 84 patients by FISH. Overall, TOP2A amplification was detected in tumor samples from four patients (4.7 %). Correlative analyses of HER2 status performed on a consecutive section demonstrated co-amplification of HER2 by FISH in one patient whose tumor was submitted to FISH analysis because of an IHC score for HER2 expression equal to 2+. HER2 overexpression (score 3+) was observed in three other patients (as per study protocol, these were not submitted to further FISH analysis since a 3+ score had already been detected by IHC). No gene deletions were detected. Discrepancies of TOP2A genomic status were detected on all paired tumor blocks submitted to FISH analysis, thereby suggesting heterogeneity of gene amplification (data not shown). The very low rate of TOP2A amplification precluded any other meaningful association with either DFS or OS.
Association of HER2 status with clinical outcome
In univariate analysis, differences based on HER2 status and survival were observed. HER2-positive patients, compared with patients whose tumors were HER2 negative, had longer DFS (at 5 years: 55.6 vs. 37.2 %, P = 0.133) and OS (at 5 years: 77.8 and 45.9 %, P = 0.051), respectively (Fig. 2). In multivariable analysis, HER2 positivity was independently associated with improved DFS [HR = 0.47 (95 % CI 0.22–0.99), P = 0.047] and OS [HR = 0.33 (95 % CI 0.13–0.83), P < 0.018] after adjusting for timing of chemotherapy/primary tumor location and disease stage (Table S2). Seven patients had HER2-positive tumors, and two (28.5 %) achieved a complete/subtotal tumor regression (tumor regression grade 1). Similar figures were observed among HER2-negative patients, where six of 25 patients (24 %) achieved complete/subtotal tumor regressions.
Discussion
We report on the analysis of HER2 and TOP2A status in a cohort of patients treated with either perioperative or postoperative epirubicin-based therapy. Consistent with previous investigations, HER2 positivity (according to the composite EMA definition) was detected in nearly 18 % of gastroesophageal adenocarcinomas, and, independently of other clinical covariates, it did correlate with improved DFS and OS. Besides disease stage, the other independent prognostic factor detected by the multivariable analysis was timing of chemotherapy, which was tightly linked to primary tumor location. The relevant prognostic value attributed to tumor location in the current study is consistent with previous reports suggesting worse outcomes for junctional primaries [2, 23] and should rule out the superiority of adjuvant chemotherapy over perioperative chemotherapy. Our findings are in substantial agreement with those reported by Yoon et al., suggesting a strong association between HER2 positivity and better survival outcomes in patients with esophageal adenocarcinoma [24]. Also, improved survival for patients with HER2 overexpression was hypothesized in the frame of the ToGA study [20].
Prognostic data on HER2 in gastroesophageal carcinomas remain somehow conflicting. A systematic review of the literature found that, among 35 published studies from 2000 to 2011, 20 (57 %) reported no difference in OS, 2 (6 %) reported significantly longer OS, and 13 studies (37 %) reported significantly poorer OS in patients with HER2 overexpression [12]. More recently, retrospective analyses did not detect survival differences according to HER2 status in three large randomized phase III trials for early-stage disease [25–27], as well as in the metastatic disease setting [28]. Despite the widespread and long-standing use of topoisomerase 2 alpha inhibitors, the predictive value of HER2 was previously examined only in the frame of the MAGIC trial [27], but there was no evidence of a differential benefit from perioperative ECF according to HER2 status. However, as pointed out by the authors [27], the rate of HER2 positivity in that study was extremely low, thereby limiting the possibility to detect a treatment-by-marker interaction. Furthermore, it is unclear whether the use of tissue microarrays (TMA) to assess HER2 expression might have flawed the overall predictive and prognostic analysis. Conversely, a retrospective investigation of the INT-0116 trial suggested that HER2 status is not prognostic, but it may identify a subset of patients who do not benefit from postoperative chemoradiation [26].
In breast cancer, the predictive role of HER2 status and TOP2A abnormalities has been challenged by controversial findings across different series [10] and by the results of a meta-analysis [29]. However, a pooled analysis of adjuvant trials indicates that both TOP2A abnormalities and/or duplication of CEP17 independently predict for anthracycline responsiveness [30], and recent attention has turned again on reappraising the role of TOP2A.
Early reports on TOP2A genomic status in HER2-amplified gastroesophageal adenocarcinomas suggested that the rate of co-amplification was even higher than in breast cancers, with a 67 % co-amplification rate among primaries arising from the cardia or the distal third of the esophagus [17]. Nevertheless, rates of TOP2A amplification in the current series were much lower and—consistent with recent investigations—our findings confirm that this phenomenon seems to be primarily linked with HER2 positivity [17, 31, 32]. Overall, TOP2A aberrations are unlikely to be a determinant of responsiveness to anthracyclines. Furthermore, despite a slightly higher rate of grade 1 regressions [19] among HER2-positive patients, our findings suggest that the supposed benefit from anthracyclines is not reflected by the histopathological tumor regression.
Assessment of HER2 expression in gastroesophageal cancers is challenged by the substantial intratumor heterogeneity of HER2 staining patterns [15, 33]. In turn, this leads to discrepancies of HER2 expression when two or more samples from the same tumor are compared. The rate of discrepancies that we observed comparing biopsies and surgical specimens for HER2 is similar to that reported by Lee et al. [15]. In published series, such rates range between 4 and 26 % [15, 34, 35]. Discrepancies of HER2 expression between biopsy and surgical specimens were not significantly different according to timing of chemotherapy, although previous investigations report on a decrease of HER2 expression in patients responding to neoadjuvant chemotherapy [35]. On the other hand, fixation artifacts should be considered, particularly when a HER2-positive status on a biopsy shifts to a negative one in a matched surgical specimen.
In addition, our results suggest that the use of small tumor biopsies may carry a significant risk to misclassify the HER2 status of a given tumor because of sampling errors. For this reason, concerns were previously raised against the use of TMA, in favor of whole tissue sections [14, 21, 36].
In our study, we elected to address the consistency of HER2 scoring by IHC in multiple blocks taken from an entire surgically resected tumor mass. To our knowledge, no study has formally addressed this issue; in fact, most reports focus on comparisons between diagnostic biopsies and paired surgical samples or surgical specimens and matched TMA. When we compared matched tumor blocks, we detected lower rates of discrepant HER2 expression levels than those we observed comparing whole tissue sections and diagnostic biopsies. Nevertheless, our investigations across three matched surgical samples from the same resection specimen imply that approximately 10 % of patients with a HER2 2+/3+ score would still be missed if the analyses were restricted to only one whole tissue section. Thus, we reaffirm the importance of retrieving a second tumor block should a negative HER2 status be detected in a first tumor block. Of note, the results of our study closely mirror those originating from the GASTHER1 study, where it has been elegantly shown that 8.7 % of HER2-negative primary tumors turn out to be HER2 positive upon repeat endoscopic biopsy [37]. These observations are relevant especially in light of the eligibility criteria for trastuzumab-based therapies [20]. Importantly, a further analysis of survival, which we performed within each set of specimens considered, confirms that only a thorough assessment of multiple samples (as opposed to a single sample) may appropriately capture the relevant information on HER2 status in patients with gastroesophageal adenocarcinomas (data not shown).
The current study has some limitations, mainly due to the lack of a treatment arm without epirubicin, to formally prove a HER2 predictive effect. However, on the basis of Chua and Merret’s report [12], despite the relatively small size of this retrospective cohort, we might speculate that the longer survival observed among HER2-positive patients might mirror a benefit from anthracycline-based chemotherapy rather than a prognostic effect of the biomarker itself.
Conclusions
We present here data that for the first time, to the best of our knowledge, demonstrate a better outcome in HER2-positive patients treated with epirubicin-based chemotherapies. In contrast, due to their low incidence, TOP2A aberrations do not appear to be major determinants of outcome. We used whole tissue sections from multiple blocks instead of TMA, thus minimizing issues related to sampling errors. We believe that this approach should be pursued to assess the predictive value of HER2 status in larger series that also include a control non-anthracycline arm.
References
Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11–20.
Ychou M, Boige V, Pignon JP, Conroy T, Bouché O, Lebreton G, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol. 2011;29:1715–21.
Kang H, Kauh JS. Chemotherapy in the treatment of metastatic gastric cancer: is there a global standard? Curr Treat Options Oncol. 2011;12:96–106.
Wagner AD, Unverzagt S, Grothe W, Kleber G, Grothey A, Haerting J, et al. Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev. 2010;3:CD004064.
Waddell T, Verheij M, Allum W, Cunningham D, Cervantes A, Arnold D. Gastric cancer: ESMO-ESSO-ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(suppl 6):vi57–63.
Ajani JA, Bentrem DJ, Besh S, D’Amico TA, Das P, Denlinger C, et al. Gastric cancer, version 2.2013: featured updates to the NCCN guidelines. J Natl Compr Cancer Netw. 2013;11:531–46.
Ajani JA, D’Amico TA, Almhanna K, Bentrem DJ, Besh S, Chao J, et al. Esophageal and esophagogastric junction cancers, version 1.2015. J Natl Compr Cancer Netw. 2015;13:194–227.
Cascinu S, Labianca R, Barone C, Santoro A, Carnaghi C, Cassano A, et al. Adjuvant treatment of high-risk, radically resected gastric cancer patients with 5-fluorouracil, leucovorin, cisplatin, and epidoxorubicin in a randomized controlled trial. J Natl Cancer Inst. 2007;99:601–7.
Fuchs CS, Tepper JE, Niedzwiecki D, Hollis D, Mamon HJ, Swanson R, et al. Postoperative adjuvant chemoradiation for gastric or gastroesophageal junction (GEJ) adenocarcinoma using epirubicin, cisplatin, and infusional (CI) 5-FU (ECF) before and after CI 5-FU and radiotherapy (CRT) compared with bolus 5-FU/LV before and after CRT: Intergroup trial CALGB 80101. Proc Am Soc Clin Oncol. 2011;29(suppl 15):4003.
Munro AF, Cameron DA, Bartlett JM. Targeting anthracyclines in early breast cancer: new candidate predictive biomarkers emerge. Oncogene. 2010;29:5231–40.
Järvinen TA, Tanner M, Rantanen V, Bärlund M, Borg A, Grénman S, et al. Amplification and deletion of topoisomerase II alpha associate with ErbB-2 amplification and affect sensitivity to topoisomerase II inhibitor doxorubicin in breast cancer. Am J Pathol. 2000;156:839–47.
Chua TC, Merrett ND. Clinicopathologic factors associated with HER2-positive gastric cancer and its impact on survival outcomes—A systematic review. Int J Cancer. 2012;130:2845–56.
Hofmann M, Stoss O, Shi D, Büttner R, van de Vijver M, Kim W, et al. Assessment of a HER2 scoring system for gastric cancer: results from a validation study. Histopathology. 2008;52:797–805.
Bilous M, Osamura RY, Rüschoff J, van de Vijver M, Hanna W, Penault-Llorca F, et al. HER-2 amplification is highly homogenous in gastric cancer. Hum Pathol. 2010;41:304–5.
Lee S, de Boer WB, Fermoyle S, Platten M, Kumarasinghe MP. Human epidermal growth factor receptor 2 testing in gastric carcinoma: issues related to heterogeneity in biopsies and resections. Histopathology. 2011;59:832–40.
Tominaga N, Gotoda T, Hara M, Hale MD, Tsuchiya T, Matsubayashi J, et al. Five biopsy specimens from the proximal part of the tumor reliably determine HER2 protein expression status in gastric cancer. Gastric Cancer. 2015. doi:10.1007/s10120-015-0502-3.
Tanner M, Hollmén M, Junttila TT, Kapanen AI, Tommola S, Soini Y, et al. Amplification of HER-2 in gastric carcinoma: association with Topoisomerase II alpha gene amplification, intestinal type, poor prognosis and sensitivity to trastuzumab. Ann Oncol. 2005;16:273–828.
Sobin LH, Gospodarowicz M, Wittekind C. TNM classification of malignant tumours. 7th ed. Oxford: Wiley-Blackwell; 2009.
Becker K, Langer R, Reim D, Novotny A, Meyer zum Buschenfelde C, Engel J, et al. Significance of histopathological tumor regression after neoadjuvant chemotherapy in gastric adenocarcinomas: a summary of 480 cases. Ann Surg. 2011;2011(253):934–9.
Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–97.
Rüschoff J, Hanna W, Bilous M, Hofmann M, Osamura RY, Penault-Llorca F, et al. HER2 testing in gastric cancer: a practical approach. Mod Pathol. 2012;25:637–50.
O’Malley FP, Chia S, Tu D, Shepherd LE, Levine MN, Bramwell VH, et al. Topoisomerase II alpha and responsiveness of breast cancer to adjuvant chemotherapy. J Natl Cancer Inst. 2009;101:644–50.
Liu K, Zhang W, Chen X, Chen X, Yang K, Zhang B, et al. Comparison on clinicopathological features and prognosis between esophagogastric junctional adenocarcinoma (Siewert II/III Types) and distal gastric adenocarcinoma: retrospective cohort study, a Single Institution, High Volume Experience in China. Medicine (Baltimore). 2015;94(34):e1386.
Yoon HH, Shi Q, Sukov WR, Wiktor AE, Khan M, Sattler CA, et al. Association of HER2/ErbB2 expression and gene amplification with pathologic features and prognosis in esophageal adenocarcinomas. Clin Cancer Res. 2012;18:546–54.
Terashima M, Kitada K, Ochiai A, Ichikawa W, Kurahashi I, Sakuramoto S, et al. Impact of expression of human epidermal growth factor receptors EGFR and ERBB2 on survival in stage II/III gastric cancer. Clin Cancer Res. 2012;18:5992–6000.
Gordon MA, Gundacker HM, Benedetti J, Macdonald JS, Baranda JC, Levin WJ, et al. Assessment of HER2 gene amplification in adenocarcinomas of the stomach or gastroesophageal junction in the INT-0116/SWOG9008 clinical trial. Ann Oncol. 2013;24:1754–61.
Okines AF, Thompson LC, Cunningham D, Wotherspoon A, Reis-Filho JS, Langley RE, et al. Effect of HER2 on prognosis and benefit from peri-operative chemotherapy in early oesophago-gastric adenocarcinoma in the MAGIC trial. Ann Oncol. 2013;24:1253–61.
Janjigian YY, Werner D, Pauligk C, Steinmetz K, Kelsen DP, Jäger E, et al. Prognosis of metastatic gastric and gastroesophageal junction cancer by HER2 status: a European and USA International collaborative analysis. Ann Oncol. 2012;23:2656–62.
Di Leo A, Desmedt C, Bartlett JM, Piette F, Ejlertsen B, Pritchard KI, et al. HER2 and TOP2A as predictive markers for anthracycline-containing chemotherapy regimens as adjuvant treatment of breast cancer: a meta-analysis of individual patient data. Lancet Oncol. 2011;12:1134–42.
Bartlett JM, McConkey CC, Munro AF, Desmedt C, Dunn JA, Larsimont DP, et al. Predicting anthracycline benefit: TOP2A and CEP17—not only but also. J Clin Oncol. 2015;33:1680–7.
Kanta SY, Yamane T, Dobashi Y, Mitsui F, Kono K, Ooi A. Topoisomerase IIalpha gene amplification in gastric carcinomas: correlation with the HER2 gene. An immunohistochemical, immunoblotting, and multicolour fluorescence in situ hybridization study. Hum Pathol. 2006;37:1333–43.
Liang Z, Zeng X, Gao J, Wu S, Wang P, Shi X, et al. Analysis of EGFR, HER2, and TOP2A gene status and chromosomal polysomy in gastric adenocarcinoma from Chinese patients. BMC Cancer. 2008;8:363.
Yang J, Luo H, Li Y, Li J, Cai Z, Su X, et al. Intratumoral heterogeneity determines discordant results of diagnostic tests for human epidermal growth factor receptor (HER) 2 in gastric cancer specimens. Cell Biochem Biophys. 2012;62:221–8.
Wang T, Hsieh ET, Henry P, Hanna W, Streutker CJ, Grin A. Matched biopsy and resection specimens of gastric and gastroesophageal adenocarcinoma show high concordance in HER2 status. Hum Pathol. 2014;45:970–5.
Watson S, Validire P, Cervera P, Zorkani N, Scriva A, Lemay F, et al. Combined HER2 analysis of biopsies and surgical specimens to optimize detection of trastuzumab-eligible patients in eso-gastric adenocarcinoma: a GERCOR study. Ann Oncol. 2013;24:3035–9.
Warneke VS, Behrens HM, Böger C, Becker T, Lordick F, Ebert MP, et al. Her2/neu testing in gastric cancer: evaluating the risk of sampling errors. Ann Oncol. 2013;24:725–33.
Park SR, Park YS, Ryu MH, Ryoo BY, Woo CG, Jung HY, et al. Extra-gain of HER2-positive cases through HER2 reassessment in primary and metastatic sites in advanced gastric cancer with initially HER2-negative primary tumours: Results of GASTric cancer HER2 reassessment study 1 (GASTHER1). Eur J Cancer. 2016;53:42–50.
Acknowledgments
This work was supported by an Internal Clinical-Translational Grant from Humanitas Clinical and Research Center to Dr. Nicola Personeni.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions. Informed consent or a substitute for it was obtained from all patients for being included in the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Personeni, N., Baretti, M., Bozzarelli, S. et al. Assessment of HER2 status in patients with gastroesophageal adenocarcinoma treated with epirubicin-based chemotherapy: heterogeneity-related issues and prognostic implications. Gastric Cancer 20, 428–437 (2017). https://doi.org/10.1007/s10120-016-0625-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10120-016-0625-1