Abstract
Background
Cancer stem cells (CSCs) have enhanced mechanisms of protection from oxidative stress. A variant form of CD44 (CD44v), a major CSC marker, was shown to interact with xCT, a subunit of cystine–glutamate transporter, which maintains high levels of intracellular reduced glutathione (GSH) which defend the cell against oxidative stress. Sulfasalazine (SSZ) is an inhibitor of xCT and was shown to suppress the survival of CD44v-positive stem-like cancer cells both in vitro and in vivo. To find the dose of SSZ which can safely reduce the population of CD44v-positive cells in tumors, a dose-escalation study in patients with advanced gastric cancer was conducted.
Methods
SSZ was given four times daily by oral administration with 2 weeks as one cycle. Tumor biopsies were obtained before and after 14 days of administration of SSZ to evaluate expression of CD44v and the intratumoral level of GSH.
Results
Eleven patients were enrolled and received a dosage from 8 to 12 g/day. Safety was confirmed up to a dosage of 12 g/day, which was considered the maximum tolerated dose. Among the eight patients with CD44v-positive cells in their pretreatment biopsy samples, the CD44v-positive cancer cell population appeared to be reduced in the posttreatment biopsy tissues of four patients. Intratumoral GSH levels were also decreased in two patients, suggesting biological effectiveness of SSZ at 8 g/day or greater.
Conclusions
This is the first study of SSZ as an xCT inhibitor for targeting CSCs. Reduction of the levels of CD44v-positive cells and GSH was observed in some patients, consistent with the mode of action of SSZ in CSCs.
Similar content being viewed by others
Introduction
Cancer stem cells (CSCs) constitute a subpopulation of cancer cells that are capable of self-renewal and tumor initiation [1]. CSCs are also more resistant to conventional therapeutic interventions than are other cancer cells [2, 3], with one resistance mechanism being dependent on an enhanced ability of CSCs to protect themselves against reactive oxygen species (ROS) [4]. CD44 is a major adhesion glycoprotein for the extracellular matrix and is implicated in a wide variety of physiological and pathological processes, including tumor cell invasion and metastasis [5, 6]. It has also been identified as a cell surface marker for CSCs of various solid tumors [5]. Splice variant isoforms of CD44 (CD44v) have recently been found to interact with the xCT subunit of the cystine–glutamate exchange transporter xc−. This interaction stabilizes xCT at the cell membrane and thereby promotes the cellular uptake of cystine and the consequent synthesis of reduced glutathione (GSH) [3, 7]. CD44v-positive cancer cells are therefore resistant to oxidative stress and possess stem-like properties.
Gastric cancer cell lines with CSC characteristics have been found to express CD44v at a relatively high level [7, 8], and forced expression of CD44v8–CD44v10, but not that of the CD44 standard (CD44s) isoform, increased the tumor initiation activity of such cells in immunocompromised mice [8]. The expression of CD44v in early gastric cancer was also shown to be positively related to the risk of recurrence in patients after curative local treatment [9, 10]. In addition, the proportion of CD44v-expressing tumor cells was increased in patients with head and neck cancer who received platinum-based chemotherapy compared with those who did not, suggesting that CD44v-positive cells are resistant to such therapy [11]. A similar drug resistance mechanism may be operative in patients with gastric cancer, for whom platinum-based chemotherapy remains the standard treatment. These lines of investigation suggest that the CD44v–xCT axis is a promising target for chemoresistant CSCs in gastric cancer.
Sulfasalazine (SSZ), a commonly administered drug for ulcerative colitis or rheumatoid arthritis, is a well-characterized specific inhibitor of the xCT-mediated cystine transport and has been shown to selectively suppress the proliferation of CD44v-positive cancer cells [12, 13]. SSZ was also found to induce the phosphorylation of p38 mitogen-activated protein kinase, an indicator of increased intracellular ROS levels, and to give rise to oxidative cytotoxicity in CD44v-positive gastric cancer cells [7] as well as to prevent distant metastasis of breast cancer cells in a mouse orthotopic model [14]. We have now performed a dose-escalation study in patients with advanced gastric cancer to determine the dose of SSZ required to safely reduce the population of CD44v-positive stem-like cancer cells.
Materials and methods
Study design
The primary objective of this single-arm, open-label, dose-escalation study was to determine the dose of SSZ that is able to safely reduce CD44v expression in tumors of patients with advanced gastric cancer. Secondary objectives included evaluation of changes in the intratumoral level of GSH associated with SSZ administration, the pharmacokinetics of the drug, adverse events, response rate, duration of response, and progression-free survival. All patients were to undergo paired tumor biopsies before and 14 days after the onset of SSZ administration. The study protocol was registered in the University Hospital Medical Information Network (UMIN) Clinical Trials Registry (ID UMIN000004055).
Patient eligibility
Criteria for patient enrollment in the study included (1) the presence of histopathologically proven unresectable or recurrent gastric adenocarcinoma, (2) the presence of a tumor amenable to biopsy before and after treatment with SSZ, (3) disease progression or treatment intolerance during one or more previous lines of systemic chemotherapy for advanced gastric cancer, (4) an age of 20 years or older, (5) an Eastern Cooperative Oncology Group (ECOG) performance status of 0–2, (6) adequate bone marrow reserve (neutrophil count of at least 1,500/mm3, hemoglobin level of at least 8.0 g/dl, platelet count of at least 75,000/mm3), (7) adequate hepatic function (serum aspartate aminotransferase and alanine aminotransferase levels of less than 100 IU/l, or of less than 200 IU/l in patients with liver metastases; serum total bilirubin concentration of less than 1.5 mg/dl), and (8) adequate renal function (serum creatinine concentration of less than 2.0 mg/dl). Patients were eligible regardless of human epidermal growth factor receptor 2 (HER2) status, which was evaluated at each institution according to the HER2 scoring system for gastric cancer as described previously [15]; HER2 positivity was defined as an immunohistochemistry score of 3+ or as both an immunohistochemistry score of 2+ and an HER2 to chromosome 17 ratio of 2.2 or greater as determined by fluorescence in situ hybridization.
Patients were excluded if they met any of the following criteria: (1) a history of chemotherapy or radiotherapy within the previous 2 weeks, (2) the presence of uncontrollable bleeding, (3) the presence of a serious comorbidity, (4) a history of allergic reaction to sulfa agents, or (5) a history of ulcerative colitis or of treatment with sulfa agents or 5-aminosalicylic acid (5-ASA). All patients provided written informed consent for their participation in the study, which was approved by the appropriate institutional ethics committee of each of the four participating institutions (National Cancer Center Hospital East, The Cancer Institute Hospital of the Japanese Foundation for Cancer Research, St Marianna University School of Medicine, and Chiba Cancer Center).
Drug administration and dose-escalation procedure
Eligible patients received SSZ (500 mg; Salazopyrin; Pfizer, Karlsruhe, Germany) by oral administration four times daily at 2 or 3 g per dose (total daily dose of 8 or 12 g) over consecutive cycles of 2 weeks until disease progression or the development of intolerable toxicity. The dosage of 8 g/day was based on the commonly accepted maximum dose in clinical practice for the treatment of ulcerative colitis [16]. In addition, on the basis on a previous pharmacokinetic study of SSZ in healthy Japanese individuals [17], this dosage was expected to yield an area under the curve similar to or greater than that (865.1 ± 33.4 μg h/ml) which was achieved by intraperitoneal infusion of SSZ at 250 mg/kg in mice and which was found to have antitumor activity [11]. Dose-limiting toxicity (DLT) was evaluated from the initial dose to the end of cycle 1. It was defined as any of the following adverse events judged to be caused by SSZ treatment: hematologic toxicity (neutropenia, leukocytopenia, anemia, thrombocytopenia) of grade 4; uncontrollable nonhematologic toxicity of grade 3 or higher despite maximal supportive care; toxicity that reduced adherence to SSZ treatment to less than 70 % of the planned dose; and toxicity that resulted in interruption of SSZ administration for a consecutive period of 14 days or longer. The dose level was escalated according to a typical 3 + 3 design. Treatment was to be temporarily discontinued after a DLT in cycle 1, possibly leading to a subsequent dose reduction or discontinuation.
The data center of the Exploratory Oncology Research and Clinical Trial Center at the National Cancer Center Hospital, Japan confirmed patient eligibility, and the dose level was then assigned. Data collection, data analysis, and data interpretation were also performed by the Exploratory Oncology Research and Clinical Trial Center at the National Cancer Center Hospital, Japan (study number EPOC1205).
Safety assessment
Adverse events were evaluated throughout the treatment period until 30 days after the last dose according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.0) for patients who received at least one dose of SSZ. Safety evaluations were based on medical review of adverse events as well as vital sign measurements, physical examination, Eastern Cooperative Oncology Group performance status, and the findings of clinical laboratory tests.
Evaluation of CD44v expression in tumor specimens
The expression status of CD44v in tumor tissue was evaluated by immunohistochemistry. Tissue sections were depleted of paraffin, rehydrated, and heated for 10 min at 105 °C in citrate buffer (10 mM, pH 6.0) for antigen retrieval before exposure to a rat monoclonal antibody specific for CD44v containing a sequence encoded by variant exon 9 [7, 11]. Areas containing predominantly cancer cells were marked by a pathologist, and the percentage of CD44v-positive cancer cells in such areas was then quantified with the use of a TissueFAXS system and the HistoQuest software program (Tissue Gnostics, Vienna, Austria).
Determination of intratumoral GSH level
The intratumoral abundance of GSH was determined with the use of a boron-doped diamond microelectrode as described previously [18]. The microelectrode needle, silver wire, and Ag/AgCl wire were inserted into the tissue to be analyzed to a depth of between 2 and 3 mm. The current was monitored in nanoamperes by chronoamperometry measurements at 1.3 V (vs Ag/AgCl).
Pharmacokinetic analysis
For pharmacokinetic analysis, the first dose of SSZ on day 1 was administered orally together with 200 ml of water after patients had fasted overnight, with a meal being permitted after blood sampling at 4 h after drug administration. Blood samples were obtained before and at 2, 3, 4, 5, and 6 h after SSZ administration in patients who had not undergone gastrectomy, or before and at 1, 2, 3, 4, and 6 h after drug administration in those who had undergone gastrectomy since the time to peak concentration is shortened generally because of the change of gastric emptying rate.
The plasma concentration of SSZ was determined with the use of an ultraperformance liquid chromatography and tandem mass spectrometry method developed specifically for this study by modification of a previously reported method [19]. The detailed experimental scheme is described in the supplementary electronic material.
Genotyping of ABCG2 and NAT2
The single nucleotide polymorphism rs2231142 in ABCG2 (421C>A, Q141K) and NAT2 genotype (NAT2*4, NAT2*5B, NAT2*6A, NAT2*7B), both of which affect exposure to SSZ, were evaluated by direct sequencing. The detailed experimental scheme of the direct sequencing is described in the electronic supplementary material.
Tumor response and statistical analysis
Tumor response was evaluated according to the Response Evaluation Criteria in Solid Tumors (version 1.1), and progression-free survival was assessed for each patient who received at least one dose of SSZ. Tumor measurements were obtained by computed tomography at the baseline, every 4 weeks for 3 months, and every 8 weeks thereafter.
All patients who received SSZ were included in the analysis of safety and efficacy. Statistical analysis of safety and efficacy was performed with the use of SAS release 9.3 (SAS Institute, Cary, NC, USA).
Results
Patient characteristics
Eleven patients at four institutions were enrolled in the study between April and December 2013 (Table 1). Six and five patients were assigned to dose level 1 (8 g/day) and dose level 2 (12 g/day) respectively. Since two of five patients in the dose level 2 group experienced DLT, we added an additional three patients (total six patients) to the dose level 1 group. One patient (patient 9) had a history of distal gastrectomy. Four patients had a positive HER2 status, all of whom had been treated with trastuzumab.
Tolerability and adverse events
Nine patients discontinued use of SSZ because of disease progression, and the remaining two patients discontinued the treatment as a result of an SSZ-related adverse event (anorexia). The median number of treatment cycles was 2 (range 1–4). The mean relative dose intensity of cycle 1 was 85 % for level 1 and 60 % for level 2, with anorexia or nausea being the commonest reasons for dose interruption. Two patients treated at dose level 2 (patients 5 and 8) experienced anorexia of grade 3 as a DLT. No patient treated at dose level 1 experienced a DLT. The commonest adverse events during all treatment cycles were gastrointestinal and hematologic toxicities (Table 2). Nausea or anorexia developed in four (67 %) and three (50 %) of the six patients at dose level 1 respectively, with most of these events being of grade 2 or lower. One patient treated at dose level 1 experienced anorexia of grade 3 associated with disease progression after completion of cycle 1. One patient treated at dose level 1 (patient 11) was associated with less than 70 % of dose intensity in cycle 1 due to lack of patient adherence rather than toxicity, and it was finally judged as a non-DLT case by the steering committee.
Changes in intratumoral CD44v expression and GSH level
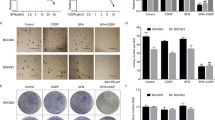
Immunohistochemical staining of tumor biopsy specimens revealed that the proportion of CD44v-positive cells was greater than 10 % in eight patients (patients 1, 2, 4, and 6–10) before SSZ treatment (Fig. 1, Table 3). Although the sample size was small, patients with HER2-positive tumors (patients 1, 9, and 10) tended to have a higher proportion of CD44v-positive cells (median of 76 %) than HER2-negative patients (median of 17 %). Tumor biopsy was not performed for patient 5. Among the eight patients with more than 10 % CD44v-positive cancer cells before SSZ treatment, the proportion of these cells was reduced by more than 10 percentage points in four patients (patients 1, 4, 9, and 10) after the treatment. The intratumoral level of GSH declined after administration of SSZ in six patients (patients 1, 3, and 6–9). The proportion of CD44v-positive cells was reduced by more than 10 percentage points and the intratumoral GSH level declined after SSZ treatment in two patients (patients 1 and 9).
Representative patient with gastric cancer who showed a reduction of the proportion of CD44v-positive cancer cells after sulfasalazine (SSZ) treatment. Patient 9 was a 64-year-old man with human epidermal growth factor receptor 2 (HER2)-positive gastric carcinoma who was treated initially with the combination of capecitabine, cisplatin, and trastuzumab and then with the combination of paclitaxel, trastuzumab, and irinotecan. The upper panels show immunohistochemical staining of tumor sections for CD44 variant (CD44v) before and after subsequent treatment with SSZ (scale bar 500 μm). The lower panels show that the proportion of CD44v-positive cells was reduced from 76 to 44 % after SSZ treatment, as indicated by the HistoQuest plots of the intensity of diaminobenzidine staining (CD44v intensity) and DNA staining. Cells with a mean intensity of more than 27 were defined as CD44v positive
Pharmacokinetics of SSZ
The maximal plasma concentration (C max) of SSZ after administration of the first dose on day 1 at level 1 and level 2 ranged from 14.0 to 46.5 μg/ml and from 10.8 to 120.2 μg/ml respectively (Table 3). The area under the time–concentration curve from 0 to 6 h (AUC0–6) after the first dose on day 1 at level 1 and level 2 ranged from 37.5 to 191.4 μg h/ml and from 30.6 to 481.9 μg h/ml respectively. The extremely high exposure to SSZ in patient 7 (C max and AUC0–6 of 120.2 μg/ml and 481.9 μg h/ml respectively) was likely attributable to the AA and NAT2*6A/*6A genotypes for ABCG2 and NAT2 respectively, which encode a transporter and a metabolic enzyme related to SSZ pharmacokinetics [17].
Tumor response
Eight of the 11 patients enrolled in the study had at least one measurable lesion. Three patients achieved unconfirmed stable disease with a duration of 49 and 28 days (patients 7 and 11 respectively), six patients had progressive disease, and two patients were not evaluable for response because of clinically determined disease progression. Median progression-free survival was not calculated because only two patients achieved stable disease.
Discussion
Repositioning of the FDA-approved drug SSZ as a cancer stem cell targeting agent might be a cost- and time-effective approach since we can use the existing drug and its safety information. We have evaluated the mode of action, tolerated dose, and pharmacokinetics of SSZ for patients with advanced gastric cancer. As far as we are aware, this is the first study to evaluate the effect of SSZ on CD44v-positive CSCs in humans. The proportion of CD44v-expressing tumor cells was found to decrease substantially after SSZ administration in four of the eight patients with CD44v-positive gastric cancer, including three individuals treated at dose level 1 (8 g/day). The intratumoral level of GSH also declined after SSZ treatment in six patients, with two patients showing downregulation of both CD44v expression and GSH abundance, consistent with the mode of action of SSZ in CSCs.
Cancer cells are often exposed to high levels of ROS derived from intrinsic or extrinsic sources [3]. Although moderate levels of ROS promote cell proliferation, high levels of ROS induce cell death. CSCs were shown to possess an enhanced antioxidant defense capability compared with non-CSCs, leading to resistance to therapeutic interventions which generate ROS. GSH plays a major role in redox adaptation in cancer cells. Cysteine, which is required for GSH biosynthesis, is normally produced through the transsulfuration pathway. However, cystine import via the xCT cystine–glutamate antiporter has recently been found as an additional source for maintaining intracellular cysteine levels. We have found that this cystine import is the major mechanism for GSH synthesis in CSCs expressing CD44v, which stabilizes the xCT transporter on the cell surface [7]. Given that SSZ inhibits the xCT-mediated cystine incorporation, selective death in CD44v-positive CSCs can be expected.
Among the five patients treated with SSZ at dose level 2 (12 g/day) in the present study, two individuals developed anorexia of grade 3 as a DLT. But a significantly decreased proportion of CD44v-positive cells was found in four patients (patients 1, 4, 9, and 10), including three patients treated a dose level 1 (8 g/day). Therefore, an SSZ dosage of 8 g/day can sufficiently reduce the proportion of CD44v-positive cells in tumors and, thereby, we can determine that the required systemic exposure to SSZ, AUC0–6, to achieve a significant reduction of the proportion of CD44v-positive cells is 30.6 μg h/ml or greater. However, three patients (patients 2, 6, and 8) with CD44v positivity (more than 10 %) and systemic exposure, AUC0–6 of 30.6 μg h/ml or greater, did not show a reduction of the proportion of CD44v-positive cells. Thus, the tumors in these patients might have additional factors that suppress the effect of CSC-targeted therapy despite their full systemic exposure to SSZ. For instance, as described above, cysteine for GSH biosynthesis is provided through not only the CD44v–xCT pathway but also the transsulfuration pathway, which is not suppressed by SSZ. Accordingly, SSZ treatment may not increase ROS levels sufficiently for complete eradication of CD44v-positive cells. Consistent with this notion, not all patients in the present study whose tumors manifested downregulation of CD44v expression after SSZ administration also showed a decrease in intratumoral GSH level. Indeed, our previous preclinical study showed that the combination of cisplatin, which triggers the generation of ROS, and SSZ induced a greater level of tumor cell death and tumor growth suppression compared with SSZ alone [7].
In patients with low expression of CD44v before the study, such as patients 6 and 8, its expression was not reduced by SSZ. Responders to SSZ might require some threshold percentage of CD44v-positive cells before treatment since the percentage of CD44v-positive cells before therapy in nonresponders was relatively low (patients 6–8).
A reduction of the proportion of CD44v-positive cells in gastric tumors did not translate into an apparent reduction in tumor size or prolongation of disease stabilization, however, considering that only two evaluable patients achieved stable disease and this effect was of short duration. Also, this observation is consistent with the findings of our previous study in which SSZ monotherapy induced the suppression of tumor growth rather than complete regression of tumors in a xenograft model [7]. Since SSZ is considered to selectively inhibit the antioxidant system which is selectively activated in chemoresistant CD44v-positive cells without affecting the chemosensitive CD44-low or CD44-negative cells, combination therapy with SSZ and ROS-generating cytotoxic anticancer agents may be required for tumor shrinkage.
In addition, given that SSZ targets the CD44v–xCT axis, which operates predominantly in a stem-like subpopulation of tumor cells, it is not effective for cancer cells that are not dependent on this axis. We were able to evaluate only a small amount of biopsied tumor tissue in the present study, and so were not able to take into account possible tumor heterogeneity. Simultaneous evaluation of whole tumor status, such as by imaging of xCT or CD44v with positron emission tomography [20], may provide more insight in future studies.
Although our analysis is limited by the small number of patients, those with HER2-positive tumors, who had already received HER2-targeted therapy, tended to show a higher proportion of CD44v-positive cancer cells that did those with HER2-negative tumors. CD44v-negative cell lines derived from head and neck squamous cell carcinoma were previously found to manifest higher levels of epidermal growth factor receptor (EGFR) signaling compared with CD44v-positive cell lines and to rely on EGFR activity for their survival [11]. In contrast, forced CD44v expression in such cells enhanced xCT dependency and reduced EGFR dependency, resulting in increased resistance to an EGFR inhibitor. Furthermore, combined treatment with SSZ and an EGFR inhibitor resulted in a synergistic reduction in tumor growth in a mouse xenograft model of EGFR-expressing head and neck squamous cell carcinoma [11]. Thus, xCT-targeted therapy may deplete CD44v-expressing head and neck squamous cell carcinoma cells, with the remaining CD44v-negative cells being sensitive to EGFR-targeted therapy. A similar mechanism might operate for treatment of HER2-positive gastric cancer with xCT- and HER2-targeted agents. Further studies with larger numbers of patients are thus warranted to evaluate the relation between CD44v expression and HER2 expression as well as the mechanism of resistance to HER2-targeted therapy in gastric cancer.
After oral administration, only about one third of the dose of SSZ is absorbed. In the intestine, the remaining SSZ is metabolized by intestinal bacteria to 5-ASA and sulfapyridine, which have no effect on xCT. Sulfapyridine is relatively well absorbed from the intestine and further metabolized by N-acetyltransferase 2 in the liver, but 5-ASA is much less well absorbed [16]. 5-ASA is an active form for treatment of ulcerative colitis, whereas sulfapyridine causes agranulocytosis, aplastic anemia, or other blood dyscrasias. Therefore, orally administered SSZ produces unwanted metabolites for xCT-targeted therapy. Furthermore, exposure to SSZ following oral administration differs markedly among individuals in a manner dependent on the ABCG2 and NAT2 genotype, as evidenced in particular in the present study by the pronounced exposure apparent in patient 7. An xCT inhibitor with less interindividual variability in exposure would thus be desirable from the clinical standpoint.
This study has several limitations: First, we did not predefine the definition of reduction of the proportion of CD44v-positive cells or GSH level before the study. Second, the small sample size in this study is a major limitation, an thus additional studies are necessary to evaluate the impact of each biomarker as well as their correlation with the pharmacokinetics.
In conclusion, we have shown that administration of SSZ at a dosage of 8 g/day in patients with advanced gastric cancer induced reduction of the proportion of CD44v-positive cells and GSH abundance in tumor cells, consistent with the mode of action of this drug in CSCs. Combination chemotherapy with SSZ and either cytotoxic agents that induce oxidative stress or inhibitors of growth factor signaling warrants further evaluation. Indeed, a study to evaluate SSZ in combination with cisplatin in previously treated patients with CD44v-expressing gastric cancer is ongoing (EPOC1407).
References
Clevers H. The cancer stem cell: premises, promises and challenges. Nat Med. 2011;17:313–9.
Trachootham D, Alexandre J, Huang P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat Rev Drug Discov. 2009;8:579–91.
Nagano O, Okazaki S, Saya H. Redox regulation in stem-like cancer cells by CD44 variant isoforms. Oncogene. 2013;32:5191–8.
Diehn M, Cho RW, Lobo NA, Kalisky T, Dorie MJ, Kulp AN, et al. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature. 2009;458:780–3.
Ponta H, Sherman L, Herrlich PA. CD44: from adhesion molecules to signalling regulators. Nat Rev Mol Cell Biol. 2003;4:33–45.
Nagano O, Saya H. Mechanism and biological significance of CD44 cleavage. Cancer Sci. 2004;95:930–5.
Ishimoto T, Nagano O, Yae T, Tamada M, Motohara T, Oshima H, et al. CD44 variant regulates redox status in cancer cells by stabilizing the xCT subunit of system xc− and thereby promotes tumor growth. Cancer Cell. 2011;19:387–400.
Lau WM, Teng E, Chong HS, Lopez KA, Tay AY, Salto-Tellez M, et al. CD44v8–10 is a cancer-specific marker for gastric cancer stem cells. Cancer Res. 2014;74:2630–41.
Hirata K, Suzuki H, Imaeda H, Matsuzaki J, Tsugawa H, Nagano O, et al. CD44 variant 9 expression in primary early gastric cancer as a predictive marker for recurrence. Br J Cancer. 2013;109:379–86.
Go SI, Ko GH, Lee WS, Kim RB, Lee JH, Jeong SH, et al. CD44 variant 9 serves as a poor prognostic marker in early gastric cancer, but not in advanced gastric cancer. Cancer Res Treat. 2015. doi:10.4143/crt.2014.227.
Yoshikawa M, Tsuchihashi K, Ishimoto T, Yae T, Motohara T, Sugihara E, et al. xCT inhibition depletes CD44v-expressing tumor cells that are resistant to EGFR-targeted therapy in head and neck squamous cell carcinoma. Cancer Res. 2013;73:1855–66.
Chen RS, Song YM, Zhou ZY, Tong T, Li Y, Fu M, et al. Disruption of xCT inhibits cancer cell metastasis via the caveolin-1/β-catenin pathway. Oncogene. 2009;28:599–609.
Zhang W, Trachootham D, Liu J, Chen G, Pelicano H, Garcia-Prieto C, et al. Stromal control of cystine metabolism promotes cancer cell survival in chronic lymphocytic leukaemia. Nat Cell Biol. 2012;14:276–86.
Yae T, Tsuchihashi K, Ishimoto T, Motohara T, Yoshikawa M, Yoshida G, et al. Alternative splicing of CD44 mRNA by ESRP1 enhances lung colonization of metastatic cancer cell. Nat Commun. 2012;3:883.
Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2015;376:687–97.
Salazopyrin summary of product characteristics. http://emc.medicines.org.uk/emc/assets/c/html/DisplayDoc.asp?DocumentID=3344. Accessed 25 Feb 2014.
Yamasaki Y, Ieiri I, Kusuhara H, Sasaki T, Kimura M, et al. Pharmacogenetic characterization of SSZ disposition based on NAT2 and ABCG2 (BCRP) gene polymorphisms in humans. Clin Pharmacol Ther. 2008;84:95–103.
Fierro S, Yoshikawa M, Nagano O, Yoshimi K, Saya H, Einaga Y. In vivo assessment of cancerous tumors using boron doped diamond microelectrode. Sci Rep. 2012;2:901.
Gu GZ, Xia HM, Pang ZQ, Liu ZY, Jiang XG, Chen J. Determination of sulphasalazine and its main metabolite sulphapyridine and 5-aminosalicylic acid in human plasma by liquid chromatography/tandem mass spectrometry and its application to a pharmacokinetic study. J Chromatogr B Analyt Technol Biomed Life Sci. 2011;879:449–56.
Baek S, Choi CM, Ahn SH, Lee JW, Gong G, Ryu JS, et al. Exploratory clinical trial of (4S)-4-(3-[18F]fluoropropyl)-l-glutamate for imaging xC − transporter using positron emission tomography in patients with non–small cell lung or breast cancer. Clin Cancer Res. 2012;18:5427–37.
Acknowledgments
This study was supported by a Health and Labor Sciences Research Grant from the Ministry of Health, Labor, and Welfare of Japan as well as by a Renovation Project of the Early and Exploratory Clinical Trial Center, National Cancer Center, Research and Development Fund (24-A-1). Study design as well as data collection, analysis, and interpretation were performed by the Exploratory Oncology Research and Clinical Trial Center, National Cancer Center, Japan. The results were previously presented in part at the Annual Meeting of the American Association for Cancer Research (San Diego, CA, USA, 5–9 April 2014), the 50th Annual Meeting of the American Society of Clinical Oncology (Chicago, IL, USA, 30 May to 3 June 2014), and the 12th Annual Meeting of the Japanese Society of Medical Oncology (Fukuoka, Japan, 17–19 July 2014).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Human rights statement and informed consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions. Informed consent or substitute for it was obtained from all patients for their being included in the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shitara, K., Doi, T., Nagano, O. et al. Dose-escalation study for the targeting of CD44v+ cancer stem cells by sulfasalazine in patients with advanced gastric cancer (EPOC1205). Gastric Cancer 20, 341–349 (2017). https://doi.org/10.1007/s10120-016-0610-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10120-016-0610-8