Abstract
Background
The incidence of gastric cancer has declined over the past decades. Little is known about trends by site and histological subtype. The aim of this study was to analyze changes in gastric cancer incidence patterns in a French well-defined population.
Methods
Data on patients with an epithelial gastric cancer diagnosed between 1982 and 2011 were collected by the population-based digestive cancer registry of Burgundy (n = 4694). Time trends in gastric cancer incidence by period of diagnosis and birth cohort were analyzed by sex, subsite, and histological type.
Results
There was a decrease in incidence rates for antral carcinomas (−2.6 % per year in males, −2.5 % per year in females; p < 0.001) and corpus carcinomas (−3.3 % and −3.2 %, respectively; p < 0.001). Annual percentage changes were not significant for fundus carcinomas in both sexes and cardia carcinoma in females, although they increased in males (+1.0 % per year; p < 0.02).When comparing the 1900 cohort and the 1950 cohort, there was a five- to sevenfold decrease in the cumulative risk at 0–79 years for corpus and antral carcinomas in both sexes and a threefold decrease for fundus carcinomas. There were minor variations for cardia carcinomas. There was a decrease of incidence both by period of diagnosis and by birth cohort for adenocarcinoma and colloid carcinoma. It was more marked for undifferentiated carcinoma. The variation for signet-ring carcinoma was minor.
Conclusion
Temporal variations in incidence rates of gastric cancer differed according to subsite and histology, suggesting different etiological factors. Available analytical studies provide an explanation for the reported trends by subsite.
Similar content being viewed by others
Introduction
Cancer of the stomach is one of the most frequent cancers in the world, with an estimated 900,000 new cases in 2012, representing 7 % of all cancers [1]. There is an approximately tenfold variation in incidence rates ranging (in males) from 60 per 100,000 in Japan and South Korea to fewer than 6 per 100,000 in the United States (USA) [2]. France stands among the low-risk countries. The incidence of stomach cancer has been declining almost everywhere for a number of years [3]. This decline was first observed in the USA and was reported later in high-risk countries [4]. Little is known about the trends in gastric cancer incidence in France. The recognition of risk factors such as Helicobacter pylori and the popularization of refrigerators, reducing salt-based preservation of food and increasing the consumption of fruits and vegetables, may explain part of the decline. Nevertheless, the overall trend may mask variable trends in incidence of gastric cancer according to subsite. This idea justifies the increasing interest in recent studies on the evolution of gastric cancer incidence by subsite, in particular the distribution between cancers originating in the cardia and those originating more distally. Moreover, few data are available about trends by histological type in spite of differences in survival according to histological type [5]. Knowledge of time trends in incidence can provide clues for understanding the role of risk or protective factors in the etiology of the disease. In particular, the effect of period of diagnosis and birth cohort may be helpful in gaining further insight as to the nature of underlying causal factors. Thus, the aim of this study is to provide trends in gastric cancer incidence, over a 30-year period, in a French well-defined population, and especially to analyze the influence of period of diagnosis and birth cohort.
Patients and methods
Study population
The population-based digestive cancer registry records all digestive tract cancer diagnosed in two administrative districts of Burgundy, France (1,052,000 in 2009). The French National Commission of Data Processing and Civil Liberty (CNIL) authorized the Registry to conduct epidemiological studies. Cancer registration began in 1976 in the Côte-d’Or area and in 1982 in the Saône-et-Loire area. Thus, the study was conducted during the 1982–2011 period. Information is routinely collected from public and private pathology laboratories, university hospitals (including the cancer center), local hospitals, private specialists (gastroenterologists, surgeons, oncologists, radiotherapists), the hospital administrative data base, the Regional Health Service records (allowing identifying patients treated outside these areas), and monthly reviews of death certificates. Cases are not recorded through death certificates alone, but these are used to identify missing cases. Registration quality and comprehensiveness is certified every 4 years by an audit performed by the National Cancer Institute (INCa), the National Institute for Health and Medical Research (INSERM), and the National Public Health Institute (InVS).
Studied variables
Subsite and morphology were coded according to the International Classification of Diseases for Oncology (ICD-03) [6]. Tumor location was divided into cardia (C16.0), fundus (upper third) (C16.1), corpus (middle third) (C16.2), antrum and pylorus (lower third) (C16.3 and C16.4), overlapping contiguous subsite (C16.8), and unspecified subsite (C16.9). Morphology codes used included adenocarcinoma (8140/3, 8260/3, 8211/3), colloid carcinoma (8480/3), signet-ring cell carcinoma (8490/3), and undifferentiated carcinomas (8020/3). In 240 cases, the diagnosis was not histologically verified but was made from operative findings or medical imaging. Nonepithelial tumors were too heterogeneous and too uncommon to analyze trends in incidence: lymphoma (n = 136), malignant endocrine tumors (n = 34), gastrointestinal stromal tumors (n = 48, identified since 2002), sarcoma (n = 27), and squamous cell carcinoma (n = 9). These categories were excluded from analysis.
Statistical analysis
The population data used for calculating incidence rates were obtained from the National Institute for Statistics and Economic Studies (INSEE). Patients were assigned to a period according to the year of diagnosis of their gastric cancer and to a to a birth cohort according to their year of birth. Six successive quinquennia from 1982–1986 to 2007–2011 and 11 successive birth cohorts were described. Each birth cohort covered 5 successive years since birth. Results started with the 1900 birth cohort, which comprised the 5 years of births from 1898 to 1902, up to the 1950 birth cohort, which comprised the years 1948–1952.
For the purpose of comparison with other countries, incidence rates were directly age-standardized to the world standard population [7]. Trends in incidence were investigated for each sex using Poisson regression with logarithmic offset, with the number of cancer cases modeled as a function of gender, age, and either calendar year or birth cohort [8]. First, for the purpose of description, annual average variations in incidence rates were estimated using a Poisson regression, adjusted for age, site, and histological type, with a linear effect of the year of diagnosis. Second, trends according to birth cohort were estimated for each sex using the best fitting age-cohort Poisson model using fractional polynomials to model the cohort effect [9]. We examined trends in the cumulative risk over the age range 0–79 years between successive birth cohorts. The cumulative risk, usually expressed as a percentage, is derived from the sum of the age-specific incidence rates [10], and it represents the cumulative probability of developing the disease over the specified age range. The advantage of the cohort cumulative risk is that the age-specific rates used to estimate it are those actually experienced by persons in that birth cohort over the part of their lifespan that has actually been observed, or modeled from the observed data. Predicted relative risks is the risk of developing gastric cancer by sex, subsite, and histological type during the age span of 0 to 79 years compared to that of the first cohort. Data were analyzed using Stata 12 software.
Results
Overall incidence
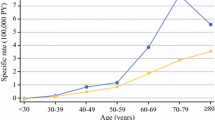
A total of 4694 epithelial gastric cancers were diagnosed between 1982 and 2011. Gastric cancer age-standardized incidence rate in males decreased from 14.9/100,000 for the 1982–1986 period to 7.4/100,000 for the 2007–2011 period. In females, the rates were 5.7 and 2.6, respectively. The average annual variation in gastric cancer incidence rates was −1.4 % (p < 0.001) in males and −1.7 % (p < 0.001) in females. The male:female ratio remained similar over time, varying between 2.6 and 3.1 (Table 1).
Table 3 plots the relative risk of developing a gastric cancer over the age range of 0 to 79 years by cohort of birth and by gender. There was a fivefold decrease in the cumulative risk between the 1900 cohort and the 1950 cohort. In males, the cumulative risk at 0–79 years decreased from 3.9 % among those born in 1900 to 0.9 % among those born in 1950. In females, it was, respectively, 1.5 % and 0.3 %.
Incidence by subsite
The time trends in incidence varied according to subsites as shown in Table 1. Between successive 5-year periods, incidence rates decreased significantly for antrum/pylorus and corpus for both genders. In contrast, there was a nonsignificant variation in incidence rates for fundus cancers in both genders. On the other hand, there was a slight increase in cardia cancer, which was significant in males, with an estimated annual increase of 1.0 % (p = 0.02). The sex ratio was higher for cardia cancers, varying between 5.0 and 7.5, than for non-cardia cancers, varying between 2.1 and 2.6.
The time trends were more homogeneous over the different subsites in cumulative risk according to birth cohort (Table 2). There was a decrease in the cumulative risk of antrum and corpus carcinomas, corresponding to a six- to sevenfold decrease for those born around 1950 compared to those born around 1900, for both males and females. The cohort effect was less important for cancers of the fundus with a threefold decrease in cumulative risk between the cohorts of 1900 and 1950. In contrast, cumulative risks by birth cohort were relatively similar over the different cohorts in males and females for cancers of the cardia.
Incidence by histological type
The time trends in incidence by histological type were similar in males and in females (Table 3). There was a significant decrease in annual incidence of 2.3 % in males and 2.8 % in females for adenocarcinoma (p < 0.001). For colloid carcinoma, it was −2.7 % in males (p = 0.03) and −4.4 % (p < 0.03) in females. The annual decrease in incidence was more pronounced for undifferentiated carcinomas, −6.1 % and −8.3 %, respectively (p < 0.001): these represented 11.7 % of gastric epithelial cancers over the 1982–1986 period and 3.6 % over the 2007–2011 period. In contrast, there was no significant variation in incidence of signet-ring cell carcinoma.
The cumulative risk of developing an adenocarcinoma of the stomach over the age range of 0 to 79 years has decreased in successive birth cohorts, corresponding to a five- to sixfold decrease in males and females (Table 4). Similar trends in variation were found for colloid carcinoma. There was a striking decrease in undifferentiated carcinomas corresponding to a 35-fold decrease in males and 100-fold decrease in females when comparing those born in 1950 to those born in 1900. There was only slight variation for signet-ring cell carcinoma in both males and females.
Discussion
Over a 30-year period, the overall incidence of gastric cancer has dramatically decreased in France. However, the most impressive aspect of our study was the diverging trends for gastric cancer incidence by subsite and histological type. The Burgundy registry is the most long-standing population-based digestive cancer registry in France. The multiplicity of information sources allows us to assume that nearly all newly diagnosed gastric cancers were recorded. When analyzing 30 years of incidence, it is necessary to evaluate the comparability of data over such a long period. The strength of our results lies in the fact that the registration scheme and the coding rules remained the same during the study period. With access to the pathological and clinical records of nearly all patients, each case could be carefully characterized. However, the diagnostic accuracy of the classification of gastric cancer by subsite has been questioned [11, 12] because of the incompleteness of subsite recording in many cancer registries. In Burgundy there has always been an interest in performing a complete and accurate classification of gastric cancer by subsite. The proportion of unknown subsite remained less than 5 % over the entire study period. Diagnosis procedure based on direct visualization of the tumor at endoscopy has not changed during the past 30 years. The endoscopy description was completed by the surgical and pathology reports. Misclassification of tumors was unlikely to explain the reported trends.
Incidence calculated by birth cohort and by period of diagnosis decreased for cancers of the intermediate and distal stomach whereas it was stable or even increasing for cancers of the cardia. For fundus cancers, decrease in incidence was seen only by cohort of birth. Our data suggest that cardia cancers have risk factors that differ from those of distal stomach cancers. Cancer registries data for the period 1980–2010 provide overall trends in incidence similar to those reported in this article in all populations except India [4]. Trends in incidence by subsite are less homogeneous. If everywhere a decrease in incidence of corpus and antral cancer is reported, incidence trends differ from one study to another for cardia cancer. Some studies report an increase in incidence in both genders [3, 13, 14] or only in males, as in our study [15, 16], and otherwise a stable incidence [17]. In some areas even a decrease in incidence is reported [18, 19]. Descriptive epidemiological data suggest that time trends in gastric cancer incidence must take into account the fact that cardia cancer and more distal gastric cancer may have different etiologies.
In many studies, the reported data are limited to the period of analysis. Time trends by birth cohort and time trends by period of diagnosis bear different implications. The cohort effect is related to exposure to early-stage risk factors or protective factors with a long-term latency period affecting each cohort of birth. A period of diagnosis effect can be attributed to risk factors or protective factors involved in the late stage of carcinogenesis affecting all age groups or to changes in screening practices. The discrepancies between cohort and case–control studies are not surprising as they are not evaluating the same phases of carcinogenesis. Initiating factors are generally different, at least in part, from promoting factors. The tendency to consider that discrepant case–control studies are not representative of the truth must be abandoned. The two types of studies are providing different information. Available data on risk factors for intermediate and distal gastric cancers allow us to interpret time trends by cohort of birth and period of diagnosis. It is well established that Helicobacter pylori is the primary risk factor for non-cardia gastric cancer [20, 21]. It is estimated that 75 % [22] to 100 % [23] of non-cardia gastric cancers worldwide were attributed, least in part to H. pylori. Helicobacter pylori infection leads to a long carcinogenic process: chronic gastritis, which may progress to atrophic gastritis, intestinal metaplasia, dysplasia, and gastric cancer [24]. The decrease in H. pylori infection in childhood for successive birth cohorts is considered as the main explanation of the observed cohort effect for distal cancer [25]. This decrease is mainly the result of better living conditions with improved hygiene and sanitation.
The decrease of salt intake over the generations can also contribute to the reported cohort effect for distal gastric cancers [26]. The protective role of vegetables and fresh fruits reported in case–control studies and not in cohort studies could be attributed to a period effect [27]. In the meta-analysis of case–control studies the relative risk for non-cardia gastric cancer was 0.61 (0.44–0.84) when comparing the highest to the lowest category of fruit consumption and 0.75 (0.59–0.95) when comparing the highest to the lowest category of vegetable consumption [28].
The slight variation in incidence for cardia gastric cancer by period of diagnosis and birth cohort suggests at least different etiological factors from more distal cancers. It is in particular the case for H. pylori infection, which is inversely associated with the risk of cardia cancer, suggesting its association with the absence of Helicobacter infection [29, 30]. Identified risk factors can explain the absence of decrease of cardia cancer incidence. Cardia cancer is associated with cigarette smoking. A meta-analysis [31] has shown a relative risk estimate of 1.9 (95 % CI, 1.3–2.7) in smokers. The role of tobacco can explain that the sex ratio is two to three times higher for cardia cancers compared to other gastric cancers. Obesity is considered as one of the main risk factor for cardia cancer. Case–control studies have consistently shown an association between high body mass index (BMI) (>30) and increased risk of cardia cancer [32–34]. A meta-analysis has shown an elevated risk for cardia gastric cancer with a relative risk of 1.4 (1.2–1.7) in overweight subjects (BMI 25–30) and 2.1 in obese subjects (BMI > 30). It has also been reported in a prospective study [35]. The protective effect of fruits and vegetables is also reported from cardia gastric cancers. A meta-analysis showed for fruits a relative risk of 0.6 (95 % CI, 0.4–0.8) when comparing the highest to the lowest category of consumption. For vegetables, it was 0.6 (95 % CI, 0.5–0.8) [28]. The protective effect of fruits and vegetables can explain that, in contrast to esophageal adenocarcinoma where obesity and tobacco consumption play a major role, incidence rates did not increase for cardia cancer.
Little is known about trends in incidence by histological subtype, apart from some data reported from Japan [36, 37]. Furthermore, it is still controversial as to which classification provides the most useful information from the epidemiological or prognostic point of view [38]. In France, pathologists usually report using the WHO classification. Other classifications such as the Lauren classification are rarely used. In this context we chose the WHO classification, which classifies gastric cancer according to microscopic morphology. The decrease in incidence of adenocarcinoma and colloid carcinoma was of similar amplitude, both by period and by birth cohort. It was more marked for undifferentiated carcinoma, which has become very rare. This result is in contrast with data from Japan indicating a more marked decrease of well-differentiated than poorly differentiated adenocarcinoma [37]. No analytical data are available to explain this difference. Contrasting with other histological types, there was no significant variation in incidence of signet-ring cell carcinoma. A similar trend was reported in Japan [36].
In conclusion, our analysis indicates that there was a dramatic decrease in gastric cancer incidence in men and in women during the past three decades and that cardia gastric cancers are epidemiologically distinct from intermediate and distal gastric cancers.
References
Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, et al. GLOBOCAN 2012 v1.0, Cancer incidence and mortality worldwide: IARC CancerBase No. 11. International Agency for Research on Cancer, Lyon, 2013. http://globocan.iarc.fr. Accessed 20 Nov 2014.
Curado M, Edwards B, Shin H, Storm H, Ferlay J, Heanue M, et al. Cancer incidence in five continents. IARC Scientific Publications no.°160, Lyon, 2007.
Wu H, Rusiecki JA, Zhu K, Potter J, Devesa SS. Stomach carcinoma incidence patterns in the United States by histologic type and anatomic site. Cancer Epidemiol Biomark Prev. 2009;18:1945–52.
de Martel C, Forman D, Plummer M. Gastric cancer: epidemiology and risk factors. Gastroenterol Clin N Am. 2013;42:219–40.
Roy P, Piard F, Dusserre-Guion L, Martin L, Michiels-Marzais D, Faivre J. Prognostic comparison of the pathological classifications of gastric cancer: a population-based study. Histopathology (Oxf). 1998;33:304–10.
WHO. International Classification of Disease for Oncology, 3rd revision. 1993; Genève.
Smith PG (1002) Cancer incidence in five continents. Comparison between registries: age-standardized rates. IARC Sci Publ 1992:865–870
Esteve J, Benhamou E, Raymond L (1994) Statistical methods in cancer research. Volume IV. Descriptive epidemiology. IARC Sci Publ 1994:1–302
Royston P, Ambler G, Sauerbrei W. The use of fractional polynomials to model continuous risk variables in epidemiology. Int J Epidemiol. 1999;28:964–74.
Day NE (1992) Cancer incidence in five continents. Cumulative rate and cumulative risk. IARC Sci Publ 1992:862–864
Camargo MC, Anderson WF, King JB, Correa P, Thomas CC, Rosenberg PS, et al. Divergent trends for gastric cancer incidence by anatomical subsite in US adults. Gut. 2011;60:1644–9.
Ekstrom AM, Signorello LB, Hansson LE, Bergstrom R, Lindgren A, Nyren O. Evaluating gastric cancer misclassification: a potential explanation for the rise in cardia cancer incidence. J Natl Cancer Inst. 1999;91:786–90.
He YT, Hou J, Chen ZF, Qiao CY, Song GH, Meng FS, et al. Trends in incidence of esophageal and gastric cardia cancer in high-risk areas in China. Eur J Cancer Prev. 2008;17:71–6.
Jezequel J, Bessaguet C, Verveur C, Faycal J, Richert Z, Metges JP, et al. Trends in incidence, management, and survival of gastric and cardia carcinomas in the area of Finistere (France) between 1984 and 2003. Eur J Gastroenterol Hepatol. 2010;22:1412–9.
Aragones N, Izarzugaza MI, Ramos M, Chirlaque MD, Almar E, Martinez C (2010) Trends in oesophago-gastric cancer incidence in Spain: analysis by subsite and histology. Ann Oncol. 2010;21(Suppl 3):69–75
Dikken JL, Lemmens VE, Wouters MW, Wijnhoven BP, Siersema PD, Nieuwenhuijzen GA, et al. Increased incidence and survival for oesophageal cancer but not for gastric cardia cancer in the Netherlands. Eur J Cancer. 2012;48:1624–32.
Dassen AE, Lemmens VE, van de Poll-Franse LV, Creemers GJ, Brenninkmeijer SJ, Lips DJ, et al. Trends in incidence, treatment and survival of gastric adenocarcinoma between 1990 and 2007: a population-based study in the Netherlands. Eur J Cancer. 2010;46:1101–10.
Coupland VH, Kocher HM, Berry DP, Allum W, Linklater KM, Konfortion J, et al. Incidence and survival for hepatic, pancreatic and biliary cancers in England between 1998 and 2007. Cancer Epidemiol. 2012;36:e207–14.
Holster IL, Aarts MJ, Tjwa ET, Lemmens VE, Kuipers EJ. Trend breaks in incidence of non-cardia gastric cancer in the Netherlands. Cancer Epidemiol. 2014;38:9–15.
Schistosomes, liver flukes and Helicobacter pylori. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Lyon, 7–14 June 1994. IARC Monogr Eval Carcinog Risks Hum 61:1–241
Biological agents. Volume 100 B. A review of human carcinogens. IARC Monogr Eval Carcinog Risks Hum 2012;100:1–441.
de Martel C, Ferlay J, Franceschi S, Vignat J, Bray F, Forman D, et al. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol. 2012;13:607–15.
Brenner H, Arndt V, Stegmaier C, Ziegler H, Rothenbacher D. Is Helicobacter pylori infection a necessary condition for noncardia gastric cancer? Am J Epidemiol. 2004;159:252–8.
Correa P. Human gastric carcinogenesis: a multistep and multifactorial process. First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 1992;52:6735–40.
Malaty HM, El-Kasabany A, Graham DY, Miller CC, Reddy SG, Srinivasan SR, et al. Age at acquisition of Helicobacter pylori infection: a follow-up study from infancy to adulthood. Lancet. 2002;359:931–5.
Wang XQ, Terry PD, Yan H. Review of salt consumption and stomach cancer risk: epidemiological and biological evidence. World J Gastroenterol. 2009;15:2204–13.
Wiseman M. The second World Cancer Research Fund/American Institute for Cancer Research expert report. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Proc Nutr Soc. 2008;67:253–6.
Lunet N, Valbuena C, Vieira AL, Lopes C, Lopes C, David L, et al. Fruit and vegetable consumption and gastric cancer by location and histological type: case-control and meta-analysis. Eur J Cancer Prev. 2007;16:312–27.
Chow WH, Blaser MJ, Blot WJ, Gammon MD, Vaughan TL, Risch HA, et al. An inverse relation between cagA+ strains of Helicobacter pylori infection and risk of esophageal and gastric cardia adenocarcinoma. Cancer Res. 1998;58:588–90.
Kamangar F, Dawsey SM, Blaser MJ, Perez-Perez GI, Pietinen P, Newschaffer CJ, et al. Opposing risks of gastric cardia and noncardia gastric adenocarcinomas associated with Helicobacter pylori seropositivity. J Natl Cancer Inst. 2006;98:1445–52.
Ladeiras-Lopes R, Pereira AK, Nogueira A, Pinheiro-Torres T, Pinto I, Santos-Pereira R, et al. Smoking and gastric cancer: systematic review and meta-analysis of cohort studies. Cancer Causes Control. 2008;19:689–701.
Kuriyama S, Tsubono Y, Hozawa A, Shimazu T, Suzuki Y, Koizumi Y, et al. Obesity and risk of cancer in Japan. Int J Cancer. 2005;113:148–57.
Sjodahl K, Jia C, Vatten L, Nilsen T, Hveem K, Lagergren J. Body mass and physical activity and risk of gastric cancer in a population-based cohort study in Norway. Cancer Epidemiol Biomark Prev. 2008;17:135–40.
Yang P, Zhou Y, Chen B, Wan HW, Jia GQ, Bai HL, et al. Overweight, obesity and gastric cancer risk: results from a meta-analysis of cohort studies. Eur J Cancer. 2009;45:2867–73.
Abnet CC, Freedman ND, Hollenbeck AR, Fraumeni JF Jr, Leitzmann M, Schatzkin A. A prospective study of BMI and risk of oesophageal and gastric adenocarcinoma. Eur J Cancer. 2008;44:465–71.
Kaneko S, Yoshimura T. Time trend analysis of gastric cancer incidence in Japan by histological types, 1975–1989. Br J Cancer. 2001;84:400–5.
Kato Y, Kitagawa T, Nakamura K, Sugano H. Changes in the histologic types of gastric carcinoma in Japan. Cancer (Phila). 1981;48:2084–7.
Berlth F, Bollschweiler E, Drebber U, Hoelscher AH, Moenig S. Pathohistological classification systems in gastric cancer: diagnostic relevance and prognostic value. World J Gastroenterol. 2014;20:5679–84.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Rights and permissions
About this article
Cite this article
Nicolas, C., Sylvain, M., Come, L. et al. Trends in gastric cancer incidence: a period and birth cohort analysis in a well-defined French population. Gastric Cancer 19, 508–514 (2016). https://doi.org/10.1007/s10120-015-0509-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10120-015-0509-9