Abstract
Background
Standard follow up for bone recurrence has not yet been established for gastric cancer after surgical resection. The aim of this study was to investigate the incidence of and related risk factors for bone recurrence after surgical resection of gastric cancer.
Methods
A cohort of 3035 gastric cancer patients after curative resection was reviewed. We analyzed the patients who had bone scintigraphy before the surgery as well as during the follow-up period. The incidence of and the risk factors for bone recurrence after surgical resection of gastric cancer were investigated.
Results
In a total of 1683 patients analyzed, bone recurrence was detected in 30 patients (1.8 %). The incidence of bone recurrence was significantly higher in advanced gastric cancers than in early lesions (3.5 vs. 0.4 %, p < 0.01). The most common recurrence site was the spine, followed by pelvic bone and rib. Most patients had multiple bone metastases. The median time for recurrence was 28 months (range 4–111) from the surgery. In univariate analysis, the recurrence rate was higher in the tumors with large size, undifferentiated pathology, location in the body, and advanced stage. In multivariate analysis, lymph node metastasis (N2/N3 vs. N0/N0I) was the most predictable risk factor for bone recurrence [hazard ratio [HR] 1.44 (95% confidence interval [CI] 1.217–1.694)] and depth of invasion (T2–4 vs. T1) was also independently associated with bone recurrence.
Conclusions
The incidence of bone recurrence was low after curative surgery in patients with gastric cancer. Intensive follow up with bone scintigraphy seems to be unnecessary in these patients.
Similar content being viewed by others
Introduction
Gastric adenocarcinoma is the most common malignancy in Korea and it is the second leading cause of cancer-related death in the world [1, 2]. Curative resection has proven to be the only successful treatment modality for locally confined gastric cancer [3]. Although the initial operation is potentially curative, a considerable proportion of patients with this tumor develop recurrent tumors [4], which indicates the importance of follow-up studies in these patients. In colorectal and breast cancers, intensive follow-up evaluation with a standardized protocol has increased the survival rate after curative surgery [5, 6]. However, the principles of follow-up evaluation have not yet been established in gastric cancer.
In one old study, bone metastasis was reported to occur in 0.7–1.4 % of gastric cancer patients [7]. Most centers performing gastric resections have unique follow-up programs influenced by past practices and experiences at the particular institution. A recent study investigated current follow-up practices after curative resection of gastric cancer using a nationwide survey in Korea, a gastric cancer endemic area [8]. That study showed a wide variety of surveillance approaches, and also revealed that half of the clinicians surveyed used bone scintigraphy as a follow-up method. Although bone recurrence has been evaluated in a variety of ways, most studies use scintigraphic methods as a screening tool, and reports have been in the form of case series or they have evaluated only small registries [9, 10]. Data regarding the value of routine bone scintigraphy in follow-up programs for curative gastrectomy have not been validated. Therefore, the aim of the present study was to investigate the incidence of and related risk factors for bone recurrence after surgical resection of gastric cancer in patients who had negative bone scintigraphy before surgery and bone scintigraphy during the follow-up period.
Subjects, materials, and methods
Subjects
Between January 1989 and December 2008, a total of 3505 consecutive patients with gastric adenocarcinoma who underwent operation at Seoul St. Mary’s Hospital, Seoul, Korea, were entered into a database, “The Gastric Cancer Patients Registry of The Catholic University of Korea”. Among these patients, 470 patients were excluded from the present analysis owing to non-curative resection: 301 patients with R1 resection (residual microscopic disease at the resection margins), 107 with R2 resection (incomplete resection with gross residual disease), and 62 with palliative surgery. Among the patients with curative gastrectomy, which was defined as “macroscopically no residual tumor and margin-negative surgical resection”, 992 patients who did not have baseline bone scintigraphy for the initial staging and 351 patients without scintigraphy during the postoperative surveillance period were excluded from the analysis. The remaining 1692 patients who had negative bone scintigraphy before surgery and bone scintigraphy during the follow-up period were eligible for the analysis. Among these 1692 patients, a second cancer developed in 9 patients; 3 with prostate cancer, 3 with tonsil cancer, 2 with colon cancer, and 1 with cholangiocarcinoma. Therefore, a total of 1683 patients were finally enrolled in our study (Fig. 1). All patients underwent total or subtotal gastrectomy with extended lymphadenectomy. The cancer was staged according to the 6th edition of the International Union Against Cancer [11]. We collected data for demographic characteristics, clinicopathologic findings, surgical modalities, and follow-up studies. This study was approved by the Institutional Review Board of Seoul St. Mary’s Hospital, Seoul, Korea.
Follow-up program
Patients with early gastric cancer (EGC) were followed up every 6 months for the first 2 years, every 12 months for the next 3 years, and every 12 months from the fifth year after surgery. For patients with advanced gastric cancer, follow-up studies were carried out every 3 months for the first 2 years, every 6 months for the next 3 years, and every 12 months from the fifth year onward. The follow-up program consisted of interim history-taking, physical examination, hematology and blood chemistry panels including tumor markers such as carcinoembryonic antigen and carbohydrate antigen 19-9, chest radiographs, abdominal computerized tomography (CT), and upper endoscopy. Patients were also examined annually with bone scintigraphy, even after recurrence in sites other than bone was detected. Magnetic resonance imaging (MRI), CT of the brain or chest, colonoscopy or barium enema, and positron emission tomography (PET)–CT were performed only when indicated. All data on the follow-up studies were evaluated on the basis of information available as of April 2010.
Bone scintigraphy
The patients were injected IV with approximately 740 MBq of Tc-99m hydroxymethylene diphosphonate (HDP). Hydration and frequent urination were encouraged. Two to three hours after the IV injection, anterior and posterior whole-body images were obtained (Siemens E.CAM, Siemens Healthcare Solutions USA, Inc., Deerfield, IL, USA). Two to four pairs of anterior and posterior spot images were additionally obtained. Pinhole images were not routinely performed.
Image review
The images and reports of all radiologic examinations including bone scintigraphy, plain films, fluoroscopy, CT and PET–CT were reviewed by a radiologist. Cases with equivocal findings were submitted to a second radiologist for review and a final interpretation was achieved by consensus.
Recurrence status in bone
Clinical records through December 31, 2008, were reviewed to identify those patients who developed local or distant osseous and non-osseous recurrence of gastric cancer. Typical presentation with multiple hot uptakes on bone scintigraphy was considered as bone metastasis. An individual scintigraphic lesion was suspected as a bone metastasis when discrete hot or cold uptake was noted. Bone recurrence was established when: (1) additional radiographic, tomographic, and/or MR images showed a typical blastic or lytic lesion, or (2) progression was seen on follow-up bone scans, with an increase in lesion intensity and appearance of at least two additional new lesions without evidence of a benign etiology. A scintigraphic abnormality which remained stable or decreased in intensity without therapy on follow-up scan was considered nonmalignant. The following findings were excluded [12]: (1) two or more adjacent, aligned rib lesions in a pattern indicative of trauma, (2) polyarticular increased uptake in the joints of the extremities, (3) diffuse or focal increased uptake in the appendicular skeleton at sites of documented trauma, and (4) increased uptake in the maxilla or mandible indicative of dental pathology.
Statistical analysis
Continuous data are presented as means ± standard deviation and categorical data are presented as quantities and proportions. To evaluate the difference between the group of patients who developed bone metastasis and the group without bone metastasis, the χ2 test for categorical data and the two-sample independent t-test were used for continuous variables. Overall survival was measured from the date of definitive surgery to the date of last follow up or death. The Kaplan–Meier method was used to plot the association curves between the time from surgery and being bone-recurrence free. To determine the risk factors affecting bone metastasis, a Cox proportional hazards model was used. In all procedures, p < 0.05 was considered the level of significance. Database management and all statistical analyses were performed using SAS software (SAS Institute, Cary, NC, USA).
Results
Characteristics of study population
The patient and tumor characteristics of the study population are shown in Table 1. The mean age was 56.4 ± 11.5 years (range 18–88). The mean tumor size was 3.9 ± 2.7 cm (range 0.1–20). The median follow-up period was 56 months. The number of patients with EGC was 937 (55.7 %).
Incidence of bone recurrence
Figure 1 shows the diagnosis of bone recurrence. In 1465 (87.0 %) of the 1683 patients, bone scintigraphy was negative for bone metastasis. Areas of abnormal radioisotope uptake were identified in the remaining 218 patients, among whom 52 patients were confirmed as having no malignant change by MRI and 136 were confirmed as having no bone metastasis by stable or decreased abnormal intensity on the follow-up bone scintigraphy. Therefore, 30 patients (1.8 %) were considered to have bone recurrence. Among these 30 patients with bone recurrence, MRI targeted to the site of abnormal uptake revealed metastases in 22 patients, CT revealed metastasis in one patient, and typical multiple metastasis was shown in 7 patients. The incidence of bone recurrence in the patients with EGC was 0.4 % (4/937) and the incidence was significantly greater, at 3.5 % (26/746), in those with advanced gastric cancer (p < 0.01, Table 1). Among the 30 patients with bone recurrence, most showed hot uptake (n = 28) on bone scintigraphy and 2 patients had cold lesions. The median time of bone recurrence from the time of gastrectomy was 28 months (range 4–111).
Sites of bone recurrence
Table 2 shows the sites of bone recurrence in the 30 patients. Twenty-five patients (83.3 %) had multiple bone recurrences. In the 5 solitary bone recurrence cases, 4 cases involved the vertebra and one case the pelvic bone. Bone recurrence developed most commonly in the spine (n = 28, 93.3 %), followed by the pelvic bone (n = 12) and ribs (n = 11). In the spine metastases, a cervical or thoracic vertebra was involved in 11 patients and a lumbar vertebra in 9. Concomitant cervical, thoracic, and lumbar involvement was found in 8 patients.
Of the 30 patients with bone recurrence, 22 (73.3 %) had concomitant recurrence in other sites, most commonly lymph node metastasis (n = 15) and carcinomatosis peritonei (n = 6). Eight patients (26.7 %) did not have nonosseous metastasis.
Risk factors for bone recurrence
Bone recurrence was more commonly found in tumors with large size, location in the body, undifferentiated pathology, deep invasion, and a higher number of metastatic lymph nodes (Table 1). Among the cancers with bone recurrence only, all had undifferentiated pathology, except for one moderately differentiated cancer. Sixty percent of tumors with bone recurrence had developed in the body, whereas tumors without bone recurrence were observed predominantly in the antrum. Bone recurrence rates were increased according to the depth of tumor invasion. According to the stage of lymph node metastasis, bone recurrence rates were 0.5, 1.1, 5.7, and 20.6 % from N0 to N3, respectively (Fig. 2).
In a Cox proportional hazard model including lymph node metastasis status, depth of invasion, and tumor location, the status of lymph node metastasis [N2/N3 vs. N0/N1; hazard ratio (HR), 1.44 (95% CI 1.217–1.694)] was the most significant factor affecting bone recurrence. Depth of invasion was also associated with bone recurrence [T2/T3/T4 vs. T1; HR 1.15 (95% CI 1.037–1.279)], whereas tumor location was not a factor (Table 3).
Bone recurrence and survival after gastrectomy
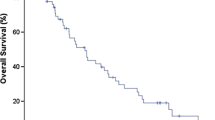
As shown in Fig. 3a, the median time of postoperative bone recurrence was significantly longer in the patients with EGC than in those with advanced gastric cancer (51 and 24 months, respectively; log-rank test, p < 0.01). The median overall survival time in the patients with bone recurrence was 35 months (range 6–112). After the diagnosis of bone recurrence, the median survival time of the patients was 6 months (range 0–49) (Fig. 3b).
Time to diagnosis of bone recurrence after surgery and patients’ survival after bone recurrence. a Interval between gastrectomy and bone recurrence. Bone recurrence developed later in the patients with early gastric cancer (EGC) than in those with advanced gastric cancer (AGC) (log-rank test, p < 0.01). b Overall survival after bone recurrence. The median survival time of the patients with bone recurrence was 6 months (range 0–49)
Discussion
The present study showed that bone recurrence developed in about 2 % of patients after the curative resection of gastric cancer. Bone recurrence occurred at a significantly higher incidence in the patients with a greater number of metastatic lymph nodes and deep tumor infiltration. As with most other tumors, bone recurrence developed in multiple sites, with the axial skeleton being the most common recurrence site.
The first aim of the present study was to assess the incidence of bone recurrence after curative surgery in gastric cancer patients. Our data showed bone recurrence developed in 1.8 % of the patients after curative resection of gastric cancer. The incidence in our study was similar to that in an old Japanese study [7]. The incidence of bone recurrence after curative gastrectomy may differ in various studies, depending on the patient population included. Our study included many patients with EGC (55.7 %), and this may have led to the low rate of bone recurrence we observed after curative gastrectomy. When the patients were grouped according to the progression of gastric cancer, the incidence was significantly higher in those with advanced lesions (3.5 %) than in the patients with EGCs (0.4 %). A previous autopsy study reported a much higher incidence of bone metastasis in patients with gastric cancer, but this could have been associated with an increase in bone metastasis at the terminal stage [13]. An old bone scan study of gastric cancer reported an incidence of bone metastasis as 46 %, but that study included patients with non-curative resection and those with baseline bone scintigraphy evaluated before surgery [14]. Of note, the patients in our study underwent extensive lymph node dissection. More radical surgery and extensive lymph node dissection may also account for the incidence of bone metastasis [15].
Bone recurrences were much more common in the axial skeleton than in the appendicular skeleton in our study. This pattern of distribution is similarly observed in the breast, prostate and lung cancer [16], and was also shown in a previous study [14]. This pattern has been explained by the correspondence to the distribution of bone marrow in an adult [17], and by venous drainage via the vertebral venous plexus, which lies outside the thoracoabdominal cavity [18]. Although our follow-up protocol included abdominal CT scans, which can also detect lower thoracic, lumbar, or sacral bone metastasis, we also found rib, skull, and sternal, as well as cervical and upper thoracic vertebral involvement, which suggests that additional bone scintigraphy is needed for the accurate assessment of bone metastasis.
Bone has been routinely checked with bone scintigraphy in many hospitals in Korea, where gastric cancer is the most common cancer [8]. However, there has been little data indicating the target patient group who would benefit from routine bone scintigraphy. Our study also aimed to find the risk factors for bone recurrence. As expected, more aggressive tumor phenotypes, including tumor size, differentiation, invasiveness, and lymph node metastasis were associated with bone recurrence in our univariate analysis. Furthermore, concomitant non-osseous recurrence with bone metastasis was observed in three-fourths of the patients. In the multivariate analysis, N2 and N3 stage had a significantly higher risk of bone recurrence than N0 and N1. As shown in Fig. 2, an initial stage with N3 node metastasis presented continuous bone recurrence during the follow-up period. Interestingly, we found that one patient who was admitted 4 months after surgery (pathologic stage, T3N3N0) because of poor general condition. This patient had back pain, which was evaluated and diagnosed as vertebral bone metastasis at this time.
A standardized protocol for postoperative follow-up evaluation in gastric cancer has not been produced. If high-intensity surveillance testing provides no improvement in duration of life or quality of life, patients can be harmed with unnecessary exposure to hazards such as radiation. Therefore, a rational follow-up strategy should be established to prevent the overuse or misuse of medical resources. It might be thought that bone scintigraphic evaluation could be beneficial in advanced gastric cancer. However, we found that the incidence of bone recurrence was very low, and only 5 of 1683 patients (0.3 %) had a solitary bone recurrence, these being the patients who would eventually benefit from a routine follow-up by bone scintigraphy. The other patients who might have become symptomatic from metastases at other sites underwent more elaborate radiologic workups. Furthermore, the survival after bone metastasis was short, as shown in Fig. 3b, which implies that the follow-up strategy with bone scintigraphy has limited prognostic value. Our study showed that little benefit can be gained by finding asymptomatic bone metastasis through routine annual follow-up with bone scintigraphy.
Two previous reports that analyzed bone recurrence in EGC and in linitis plastica support our data, in that the number of metastatic lymph nodes correlated with a probability of bone recurrence [19, 20]. Both of these studies analyzed small numbers of patients. Our results can allow generalization because a large number of patients were followed up for a long time, at repeated intervals, for bone recurrence. The present data can guide physicians in selecting patients who could benefit from bone scintigraphy evaluation during postoperative follow up.
Most metastases in gastric cancer are generally regarded as progressing sequentially from locoregional spread to distant lesions. One hypothesis of bone metastasis was that explained by progression from lymph node metastasis to hematogenous spread [21, 22]. However, our result and a previous report [23] showed that even EGC can develop bone metastasis without discernable liver or lung metastasis. Bone metastasis is considered to be of hematogenous origin through the bone marrow [24]. It is hard to find direct evidence on whether bone marrow metastasis frequently develops into bone metastasis. Our data showed that bone metastasis was frequently found in the axial skeleton, such as in the spine, pelvic bones, or sternum, which could be the areas of hematopoietic marrow in adults. Therefore, the bone marrow, rather than the bone tissue per se, seems to be the target in bone metastasis. Another possible explanation is tumor dormancy, which indicates a state of co-existence in which tumor cells persist in a clinically normal host for prolonged periods of time [25]. In bone recurrence, a hidden vascular metastasis may exist before bone involvement of cancer cells occurs. In our study, bone metastasis was found in 0.45 % of EGCs and the recurrence interval from surgery was long compared with that of advanced-stage cancer. This finding may also suggest that some bone metastases are associated with bone marrow micrometastasis existing in the state of tumor dormancy. As factors activating this hematopoietic marrow metastasis, various cytokines, such as epithelial growth factor, vascular epithelial growth factor, interleukin-1, and tumor necrosis factor-α have been proposed in previous studies [26–29].
In case of bone marrow metastasis before cortex involvement, bone scintigraphy may not be able to detect bone metastasis. The imaging techniques available for the diagnosis of bone metastasis include plain radiographs, radionuclide bone scanning, CT, and MRI. Data on the specificity and the frequency of positive scan results in the absence of metastatic disease are difficult to obtain, partly because of the methods used to validate the data. Our present data were assessed by using technetium bone scintigraphy and were evaluated with other methods. Besides CT or MRI, gallium-67 uptake bone scintigraphy can be useful to differentiate inflammatory bone lesions from bone metastasis [30].
Our study has the following strong points. First, we used bone scintigraphy, a sensitive examination for bone metastasis, as a screening tool and confirmed it with other imaging studies when the bone scintigraphy finding was not conclusive. We note that a single abnormality has a significant probability of being due to a nonmalignant lesion [31]. A single bone recurrence was found in 5 of the 30 patients in our study. To differentiate benign lesions, all patients were evaluated with MRI and 3 of them were also assessed with PET–CT. Second, a large number of patients were evaluated with bone scintigraphy to make sure of the absence of bone metastasis before surgery, and serial imaging studies were performed, followed up repetitively over a long follow-up time.
Our analysis has some limitations. Although we measured bone recurrence with a sensitive method, bone scintigraphy, the bone recurrence cases were not confirmed with pathologic diagnosis. Additionally, the present results were analyzed in a retrospective manner. Therefore, symptoms related to the bone metastasis could not be evaluated exactly. Because we found only 30 patients with bone metastasis, all factors potentially influencing bone recurrence could not be included in the multivariate analysis.
In conclusion, bone recurrence is not frequent in gastric cancer patients who undergo curative gastrectomy. Patients with advanced-stage cancer showed a significantly higher incidence of bone recurrence, and most of these patients also had combined recurrences. After the diagnosis of bone recurrence, the survival of the patients was short. Therefore, intensive follow up with bone scintigraphy seems to be unnecessary after curative gastrectomy in these patients. Our study can add information for the detection of bone recurrence after curative gastrectomy.
References
Shin HR, Jung KW, Won YJ, Park JG. 2002 Annual report of the Korea Central Cancer Registry: based on registered data from 139 hospitals. Cancer Res Treat. 2004;36:103–14.
Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108.
Song KY, Park SM, Kim SN, Park CH. The role of surgery in the treatment of recurrent gastric cancer. Am J Surg. 2008;196:19–22.
Lehnert T, Rudek B, Buhl K, Golling M. Surgical therapy for loco-regional recurrence and distant metastasis of gastric cancer. Eur J Surg Oncol. 2002;28:455–61.
Collins RF, Bekker HL, Dodwell DJ. Follow-up care of patients treated for breast cancer: a structured review. Cancer Treat Rev. 2004;30:19–35.
Jeffery M, Hickey BE, Hider PN. Follow-up strategies for patients treated for non-metastatic colorectal cancer. Cochrane Database Syst Rev. 2007:CD002200.
Yoshikawa K, Kitaoka H. Bone metastasis of gastric cancer. Jpn J Surg. 1983;13:173–6.
Hur H, Song KY, Park CH, Jeon HM. Follow-up strategy after curative resection of gastric cancer: a nationwide survey in Korea. Ann Surg Oncol. 2010;17:54–64.
Cirera L, Balil A, Batiste-Alentorn E, Tusquets I, Cardona T, Arcusa A, et al. Randomized clinical trial of adjuvant mitomycin plus tegafur in patients with resected stage III gastric cancer. J Clin Oncol. 1999;17:3810–5.
Ubukata H, Motohashi G, Tabuchi T, Nagata H, Konishi S, Tabuchi T. Overt bone metastasis and bone marrow micrometastasis of early gastric cancer. Surg Today. 2011;41:169–74.
Sobin LH, Wittekind C. International Union Against Cancer (UICC): TNM classification of malignant tumours. 6th ed. New York: Wiley-Liss; 2002.
Jacobson AF, Stomper PC, Jochelson MS, Ascoli DM, Henderson IC, Kaplan WD. Association between number and sites of new bone scan abnormalities and presence of skeletal metastases in patients with breast cancer. J Nucl Med. 1990;31:387–92.
Mori W, Adachi Y, Okabe H, Ohta K. An analysis of 755 autopsied cases of malignant tumors. A statistical study of their metastasis. Gan No Rinsho. 1963;9:351–74.
Choi CW, Lee DS, Chung JK, Lee MC, Kim NK, Choi KW, et al. Evaluation of bone metastases by Tc-99m MDP imaging in patients with stomach cancer. Clin Nucl Med. 1995;20:310–4.
Strong VE, Song KY, Park CH, Jacks LM, Gonen M, Shah M, et al. Comparison of gastric cancer survival following R0 resection in the United States and Korea using an internationally validated nomogram. Ann Surg. 2010;251:640–6.
Galasko C. The anatomy and pathways of skeletal metastases. In: Weiss L, Gilbert A, editors. Bone metastases. Boston: GK Hall; 1981. p. 49–63.
Willis RA. Secondary tumors of bone. In: The spread of tumors in the human body. London: Butterworth; 1973. p. 229–50.
Batson OV. The role of the vertebral veins in metastatic processes. Ann Intern Med. 1942;16:38–45.
Gunji Y, Suzuki T, Hori S, Hayashi H, Matsubara H, Shimada H, et al. Prognostic significance of the number of metastatic lymph nodes in early gastric cancer. Dig Surg. 2003;20:148–53.
Kodera Y, Ito S, Mochizuki Y, Yamamura Y, Misawa K, Ohashi N, et al. The number of metastatic lymph nodes is a significant risk factor for bone metastasis and poor outcome after surgery for linitis plastica-type gastric carcinoma. World J Surg. 2008;32:2015–20.
Bross IDJ, Blumenson LE. Metastatic sites that produce generalized cancer: identification and kinetics of generalized sites. In: Weiss L, editor. Fundamental aspects of metastasis. Amsterdam: North-Holland; 1976. p. 359–75.
Viadana E, Bross ID, Pickren JW. The metastatic spread of cancers of the digestive system in man. Oncology. 1978;35:114–26.
Kobayashi M, Okabayashi T, Sano T, Araki K. Metastatic bone cancer as a recurrence of early gastric cancer—characteristics and possible mechanisms. World J Gastroenterol. 2005;11:5587–91.
Abramson DI. Blood vessels and lymphatics. New York: Academic Press; 1962.
Demicheli R, Terenziani M, Valagussa P, Moliterni A, Zambetti M, Bonadonna G. Local recurrences following mastectomy: support for the concept of tumor dormancy. J Natl Cancer Inst. 1994;86:45–8.
Arguello F, Baggs RB, Graves BT, Harwell SE, Cohen HJ, Frantz CN. Effect of IL-1 on experimental bone/bone-marrow metastases. Int J Cancer. 1992;52:802–7.
Holmgren L, O’Reilly MS, Folkman J. Dormancy of micrometastases: balanced proliferation and apoptosis in the presence of angiogenesis suppression. Nat Med. 1995;1:149–53.
Kosaka Y, Mimori K, Fukagawa T, Ishikawa K, Etoh T, Katai H, et al. Identification of the high-risk group for metastasis of gastric cancer cases by vascular endothelial growth factor receptor-1 overexpression in peripheral blood. Br J Cancer. 2007;96:1723–8.
Mimori K, Fukagawa T, Kosaka Y, Kita Y, Ishikawa K, Etoh T, et al. Hematogenous metastasis in gastric cancer requires isolated tumor cells and expression of vascular endothelial growth factor receptor-1. Clin Cancer Res. 2008;14:2609–16.
Kim JG, Ryoo BY, Park YH, Kim BS, Kim TY, Im YH, et al. Prognostic factors for survival of patients with advanced gastric cancer treated with cisplatin-based chemotherapy. Cancer Chemother Pharmacol. 2008;61:301–7.
Corcoran RJ, Thrall JH, Kyle RW, Kaminski RJ, Johnson MC. Solitary abnormalities in bone scans of patients with extraosseous malignancies. Radiology. 1976;121:663–7.
Acknowledgments
This work was supported by the 2011 Seoul St. Mary’s Hospital Clinical Medicine Research Program. We would like to thank the Clinical Research Coordinating Center of Catholic Medical Center (CRCC) for statistical analysis.
Conflict of interest
None to be declared.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Park, J.M., Song, K.Y., O, J.H. et al. Bone recurrence after curative resection of gastric cancer. Gastric Cancer 16, 362–369 (2013). https://doi.org/10.1007/s10120-012-0193-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10120-012-0193-y