Abstract
Background
Adjuvant chemoradiotherapy (CRT) is the standard treatment in Western countries for gastric cancer patients submitted to curative resection. However, the role of adjuvant CRT in gastric cancer treated with D2 lymphadenectomy has not been well defined.
Methods
We conducted a retrospective study in patients with stage II to IV gastric adenocarcinoma with no distant metastases, who underwent curative resection with D2 lymphadenectomy between January 2002 and December 2007. The present study compared the 3-year overall survival of two treatments (adjuvant CRT according to the INT 0116 trial versus resection alone). Survival curves were estimated by the Kaplan–Meier method and compared with a log-rank test. Multivariate analysis of prognostic factors was performed by the Cox proportional hazards model.
Results
A total of 185 patients were included, 104 patients (56 %) received adjuvant CRT and 81 received resection alone. The 3-year overall survival was 64.4 % in the CRT group and 61.7 % in the resection-alone group (p: 0.415). However, according to the Cox proportional hazards model, adjuvant CRT was a prognostic factor for 3-year overall survival (hazard ratio [HR] 0.46, 95 % confidence interval [CI] 0.26–0.82, p: 0.008).
Conclusions
In the present study, adjuvant CRT was associated with a lower risk of death over a 3-year period in gastric cancer patients treated with D2 lymphadenectomy.
Similar content being viewed by others
Introduction
Gastric cancer is a malignant neoplasm with the fourth-highest incidence worldwide, represents the second-greatest cause of cancer-related deaths, and frequently affects the populations of Latin America, Eastern Europe, China, and Japan [1, 2].
Gastric cancer that is limited to the stomach and to regional lymph nodes is potentially curable by surgical resection. However, about 20–40 % of all patients submitted to resection with no further treatment will suffer a relapse of the disease within 2 years [3, 4]. Once a relapse occurs, no curative treatment is available and the median survival is about 6 months [3].
In view of these unfavorable outcomes of recurrent gastric cancer, adjuvant treatments have been tested during the past decade. In 2001, based on the results of the INT 0116 trial, adjuvant chemoradiotherapy with 5-fluorouracil started to be widely accepted in Western countries by demonstrating a significant benefit in overall survival [5]. However, the results of the trial were contested because only 10 % of the population studied had been submitted to D2 lymphadenectomy, which is considered to reduce the risk of locoregional recurrence and the risk of death related to gastric cancer [5, 6].
The presence of residual disease in nonresected lymph nodes, which can be measured with a quantitative estimator denoted as the Maruyama index, is an independent prognostic factor in patients with gastric cancer [7–9]. The addition of radiotherapy to adjuvant therapy can be a useful strategy in patients submitted to more limited dissections, with a higher risk of microscopic locoregional disease. In patients with more extensive dissections, radiotherapy may not be of additional benefit in terms of survival and may actually have a deleterious effect by increasing the toxicity of treatment [5, 10–12].
Recent data support the hypothesis that patients with gastric cancer benefit from adjuvant chemotherapy [13], but there is still no agreement about the therapeutic regimen to be employed. Perioperative chemotherapy or adjuvant chemotherapy alone has proven to be promising in clinical trials with predominantly D2 lymphadenectomy dissection [14–17].
The objective of the present study was to assess the influence of adjuvant chemoradiotherapy on the overall survival of patients with gastric cancer submitted to D2 lymphadenectomy.
Patients and methods
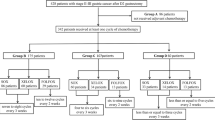
This was a retrospective cohort study conducted in patients with a histological diagnosis of stage II to IV gastric or esophagogastric junction (EGJ) adenocarcinoma, with no distant metastases, according to the classification of the American Joint Committee on Cancer 6th edition. These patients were submitted to curative resection and D2 lymphadenectomy at the Barretos Cancer Hospital (Barretos, São Paulo, Brazil) between January 2002 and December 2007.
Surgical treatment consisted of gastrectomy with free margins (R0 resection) and D2 lymphadenectomy, without splenectomy or caudal pancreatectomy. The procedure was performed by a single team of oncology surgeons with training in surgery of the digestive system. The indication for chemoradiotherapy or for resection alone was based on the clinical judgment of the oncology team, which consisted of surgical, medical, and radiation oncologists.
Adjuvant therapy consisted of five cycles of intravenous 5-fluorouracil and leucovorin (425 and 20 mg/m2, respectively, daily) for 5 days, every 28 days, concomitant with radiotherapy in cycles two and three, when the dose of 5-fluorouracil was reduced to 400 mg/m2, according to the INT 0116 trial [5]. The total radiotherapy dose was 45 Gy in 25 fractions over 5 days of the week and was applied to the tumor bed; areas of anastomosis; and perigastric, celiac, para-aortic, splenic, hepatoduodenal; and pancreatoduodenal lymph nodes. In tumors of the EGJ and of the proximal portions of the stomach, the field was proximally expanded by 3–5 cm to include the distal esophagus area and part of the left hemidiaphragm, as well as regions of paracardiac and paraesophageal lymph nodes. Anteroposterior (AP) and posteroanterior (PA) fields were used in the conventional technique, with a 6-MV linear accelerator or cobalt unit. The cardiac area was protected with blocks and at least 2/3 of one kidney was spared from irradiation.
After the end of treatment, the patients were followed up by anamnesis, physical examination, laboratory tests (blood count, hepatic function and renal function) and a chest radiograph every 3 months during the first 2 years, every 4 months during the third year, and every 6 months starting in the fourth year. Patients were also given an abdominal ultrasound every 4 months during the first 2 years and every 6 months starting in the third year. Computed tomography, nuclear magnetic resonance, and upper digestive endoscopy were performed upon clinical indication.
Recurrence was diagnosed based on clinical examination findings and on the complementary tests performed according to the above schedule, with a description of all sites diagnosed during the follow up. Recurrence at the surgical site, in the anastomosis, and in the regional lymph nodes was considered to be locoregional. Peritoneal implants or ascites with no other evident cause were considered to be signs of peritoneal recurrence. Systemic recurrence was considered if distant metastases appeared, including retroperitoneal lymph nodes. Patients with recurrences were evaluated for palliative chemotherapy.
The study compared the 3-year overall survival, as well as the rates and sites of recurrence, in the group of patients submitted to gastrectomy and D2 lymphadenectomy alone (GL) with these parameters in the group of patients submitted to adjuvant chemoradiotherapy (GL + CRT). Finally, the prognostic factors for survival were identified.
The categorical variables in the two groups were compared by the χ2 test and the continuous variables were compared by the Mann–Whitney test. Overall survival was defined as the time, in months, elapsed from the date of surgery to the date of death from any cause. The 3-year overall survival was estimated by the Kaplan–Meier method, and the survival curves were compared by the log-rank test. The patients lost to follow up were censored on the date of last contact with the center. Surviving patients were censored within 36 months for the analysis of overall survival. Prognostic factors were assessed by multivariate analysis using the Cox proportional hazards model, and p values of less than 0.05 were considered to indicate statistical significance. The analyses were performed with SPSS 13.0 software (SPSS, Chicago, IL, USA).
The study was approved by the ethics committee of the participating center.
Results
Of the 185 patients selected, 113 (61 %) were males. The median age was 61 years. The median follow up was 30.8 months. Gastric tumors represented 85 % of the cases, and the remaining tumors were those of the EGJ. All patients underwent R0 resection and D2 lymphadenectomy. Nine patients (4.6 %) died during the postoperative period and were not included in the analysis.
A total of 104 (56 %) patients were submitted to adjuvant chemoradiotherapy, and 81 were treated with resection alone. GL + CRT patients were younger and presented more advanced nodal status and TNM stage than GL patients. The characteristics of the groups are listed in Table 1. The rates and sites of recurrence and the frequency of locoregional, peritoneal, and systemic recurrence are listed in Table 2.
During the follow-up period, 68 deaths occurred, 37 in GL + CRT patients and 31 in GL patients. The 3-year overall survival rate was 63.4 %. Median survival was not reached. The 3-year overall survival rate was 64.4 % for GL + CRT and 61.7 % for GL (p: 0.415) (Table 2; Fig. 1).
The Cox proportional hazards model adjusted for age, gender, depth of invasion, nodal status, TNM stage, tumor location, Laurén histology, and adjuvant treatment revealed that only TNM stage and adjuvant treatment had influence on overall survival (Table 3). The patients treated with chemoradiotherapy had a 54 % reduction of the risk of death within 3 years (hazard ratio [HR] 0.46 95 % confidence interval [CI] 0.26–0.82; p: 0.008). The HR differed according to stage of disease (Table 4). Patients with no information about Laurén histology (11 patients) were excluded from the multivariate analysis. Only one treatment-related death occurred among GL + CRT patients. The administration of palliative chemotherapy was also similar for both groups, i.e., 16.5 % in GL + CRT and 11.3 % in GL (p: 0.313).
Discussion
The indication of adjuvant chemoradiotherapy for patients submitted to curative gastrectomy and D2 lymphadenectomy has been debated over the past few years. A retrospective study involving Eastern patients exclusively operated on with D2 lymphadenectomy showed that adjuvant treatment with combined therapy reduced the risks of recurrence and death by 20 % [18]. The ARTIST [17] and CLASSIC [16] trials recently raised the question of the benefit of adjuvant chemoradiotherapy and chemotherapy in Eastern patients submitted to D2 lymphadenectomy. On the other hand, there are no studies that have evaluated the effect of adjuvant chemoradiotherapy according to the pivotal INT 0116 trial in a Western population who have undergone D2 lymphadenectomy exclusively.
In the present study, the patients were evaluated for eligibility for adjuvant treatment after surgery and there was the possibility that postoperative complications (i.e., poor performance status, slow postoperative recovery, poor nutritional status) influenced the decision of the choice of adjuvant therapy. The limitations of this study could explain the heterogeneity of the groups in relation to age, TNM, and node stages, but the use of the regression model adjusted for well-recognized prognostic factors aimed to minimize the influence of these factors on the prognostic value of adjuvant therapy.
Based on the analysis by the regression model with a meaningful hazard ratio (HR), it was suggested that chemoradiotherapy was associated with a lower risk of death within 3 years, with a reduction of 54 %. There was a different benefit derived from adjuvant therapy according to disease stage (Table 4). Patients with stage IV disease with no distant metastases derived the greater benefit from the combined modality therapy, contributing to the hypothesis that patients with more advanced nodal disease could benefit from more intensive local therapy. It is possible that the expressive HR of 0.25 in this subgroup of patients affected the conclusions in relation to the whole population. It is also important to emphasize that such a model-based analysis imposes limitations on conclusions about reduction of the risk of events when compared with design-based analysis, which is usually performed in randomized clinical trials.
The 3-year overall survival of 61.7 % in our patients with gastrectomy and D2 lymphadenectomy alone (GL) is a satisfactory outcome, although inferior to the finding of the ACTS-GS Group trial in a patient population with D2 or D3 lymphadenectomy, which recorded a 3-year overall survival rate of 70.1 % [15]. In the present study, the classification of lymphadenectomy was done on the basis of operative reports; however, there was a wide variation in the number of dissected lymph nodes, with at least 12 in both groups, which is low for D2 resection. This observation, coupled with the presence of a higher percentage of locally advanced disease in the sample studied could explain the 3-year overall survival difference observed between the resection-alone arm of the ACTS-GC Group trial [15] and the similar arm in the present study.
The survival outcome of our group submitted to adjuvant therapy was 64.4 % and this also can be considered satisfactory. In the INT 0116 and ACTS-GS Group trials, the 3-year survival rates were 50 and 80.1 %, respectively [5, 15]. The population investigated in the latter trial presented an earlier stage of disease, with 49.9 % of the patients in stages III and IV with no distant metastases compared with 77 % of the patients in these stages in the present study.
In summary, chemoradiotherapy based on 5-fluorouracil seems to be an effective adjuvant therapy in patients with gastric and EGJ adenocarcinoma submitted to D2 lymphadenectomy, and it will probably continue to be used as one of the options of adjuvant therapy. Through the lessons learned from breast and colon cancers, in which benefits derived from adjuvant therapy have been noted to differ according to the presence of prognostic and predictive factors, and in consideration of the findings of the ARTIST [17] and ToGA [19] trials, maybe it would be interesting to examine carefully the design of more personalized randomized trials for adjuvant therapy in gastric cancer that take into account tailored therapies.
References
Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59(4):225–49.
Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55(2):74–108.
D’Angelica M, Gonen M, Brennan MF, Turnbull AD, Bains M, Karpeh MS. Patterns of initial recurrence in completely resected gastric adenocarcinoma. Ann Surg. 2004;240(5):808–16.
Yoo CH, Noh SH, Shin DW, Choi SH, Min JS. Recurrence following curative resection for gastric carcinoma. Br J Surg. 2000;87(2):236–42.
Macdonald JS, Smalley SR, Benedetti J, Hundahl SA, Estes NC, Stemmermann GN, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345(10):725–30.
Songun I, Putter H, Kranenbarg EM, Sasako M, van de Velde CJ. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol. 2010;11(5):439–49.
Hundahl SA, Macdonald JS, Benedetti J, Fitzsimmons T. Surgical treatment variation in a prospective, randomized trial of chemoradiotherapy in gastric cancer: the effect of undertreatment. Ann Surg Oncol. 2002;9(3):278–86.
Peeters KC, Hundahl SA, Kranenbarg EK, Hartgrink H, van de Velde CJ. Low Maruyama index surgery for gastric cancer: blinded reanalysis of the Dutch D1–D2 trial. World J Surg. 2005;29(12):1576–84.
Dikken JL, Jansen EP, Cats A, Bakker B, Hartgrink HH, Kranenbarg EM, et al. Impact of the extent of surgery and postoperative chemoradiotherapy on recurrence patterns in gastric cancer. J Clin Oncol. 2010;28(14):2430–6.
Bamias A, Karina M, Papakostas P, Kostopoulos I, Bobos M, Vourli G, et al. A randomized phase III study of adjuvant platinum/docetaxel chemotherapy with or without radiation therapy in patients with gastric cancer. Cancer Chemother Pharmacol. 2010;65(6):1009–21.
Schwartz GK, Winter K, Minsky BD, Crane C, Thomson PJ, Anne P, et al. Randomized phase II trial evaluating two paclitaxel and cisplatin-containing chemoradiation regimens as adjuvant therapy in resected gastric cancer (RTOG-0114). J Clin Oncol. 2009;27(12):1956–62.
Markelis R, Endzinas Z, Kiudelis M, Grizas S, Pundzius J, Saladzinskas Z, et al. Adjuvant therapy after curative resection with D2 lymphadenectomy for gastric cancer: results of a prospective clinical trial. Medicina (Kaunas). 2009;45(6):460–8.
Paoletti X, Oba K, Burzykowski T, Michiels S, Ohashi Y, Pignon JP, et al. Benefit of adjuvant chemotherapy for resectable gastric cancer: a meta-analysis. JAMA. 2010;303(17):1729–37.
Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355(1):11–20.
Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Fujii M, Nashimoto A, et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007;357(18):1810–20.
Bang YJ, Kim YW, Yang HK, Chung HC, Park YK, Lee KH, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet. 2012;379(9813):315–21.
Lee J, do Lim H, Kim S, Park SH, Park JO, Park YS, et al. Phase III trial comparing capecitabine plus cisplatin versus capecitabine plus cisplatin with concurrent capecitabine radiotherapy in completely resected gastric cancer with D2 lymph node dissection: the ARTIST trial. J Clin Oncol. 2012;30(3):268–73.
Kim S, Lim DH, Lee J, Kang WK, MacDonald JS, Park CH, et al. An observational study suggesting clinical benefit for adjuvant postoperative chemoradiation in a population of over 500 cases after gastric resection with D2 nodal dissection for adenocarcinoma of the stomach. Int J Radiat Oncol Biol Phys. 2005;63(5):1279–85.
Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376(9742):687–97.
Acknowledgments
Supported by Fundação Waldemar Barnsley Pessoa, Brazil.
Conflict of interest
None declared.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Jácome, A.A.A., Wohnrath, D.R., Scapulatempo Neto, C. et al. Effect of adjuvant chemoradiotherapy on overall survival of gastric cancer patients submitted to D2 lymphadenectomy. Gastric Cancer 16, 233–238 (2013). https://doi.org/10.1007/s10120-012-0171-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10120-012-0171-4