Abstract
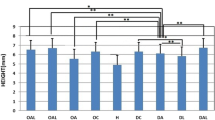
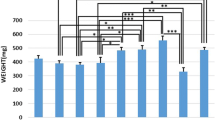
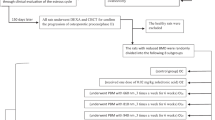
Osteoporosis (OP) is a disease which causes bone loss and fractures, leading to severe pain and deformity. This study has aimed to assess the effects of pulsed wave low-level laser therapy (PW LLLT) on cortical bone in two experimental models of OP in rats. There were four ovariectomized (OVX-d) groups and four dexamethasone-treated groups. The healthy group were considered for baseline evaluations. At 14 weeks following ovariectomy, the OVX-d rats were further subdivided into the following: control rats with OP, OVX-d rats that received alendronate (1 mg/kg), OVX-d rats treated with LLLT, and OVX-d rats treated with alendronate and LLLT. The remaining rats received dexamethasone for 5 weeks and were divided into four groups: control, alendronate-treated rats (1 mg/kg), laser-treated rats, and laser-treated rats with concomitant administration of alendronate. The rats received alendronate for 30 days. LLLT (890 nm, 80 Hz, 0.972 J/cm2) was performed on the tibias three times per week for 8 weeks. After 8 weeks, tibias were extracted and submitted to a three-point bending test. PW LLLT did not increase the biomechanical parameters of osteoporotic bones compared to controls and healthy rats. PW LLLT associated with alendronate treatment significantly increased stress high load in OVX-d rats compared to the healthy group. PW LLLT at the current study parameters failed to cause beneficial biomechanical effects in the examined osteoporotic cortical bones. PW LLLT associated with alendronate treatment produced a more remarkable effect on bone strength in the ovariectomized induced OP rat model.





Similar content being viewed by others
References
Johnell O, Kanis JA, Oden A, Sernbo I, Redlund-Johnell I, Pitterson C, de Laet C, Jonsson B (2004) Mortality after osteoporotic fractures. Osteoporos Int 15:38–42
U.S. Department of Health and Human Services (2004) Bone health and osteoporosis: a report of the Surgeon General. U.S. Department of Health and Human Services, Office of the Surgeon General, Rockville
(2009) Chapter 1: a public health approach to promote bone health. Available at: http://www.surgeongeneral.gov/library/bonehealth/chapter_1.html#TheMagnitudeoftheProblem. Accessed 14 Sept 2009
Riggs BL, Khosla S, Melton LJ 3rd (2002) Sex steroids and the construction and conservation of the adult skeleton. Endocr Rev 23:279–302
Eriksen EF, Hodgson SF, Eastell R, Cedel SL, O’Fallon WM, Riggs BL (1990) Cancellous bone remodeling in type I (postmenopausal) osteoporosis: quantitative assessment of rates of formation, resorption, and bone loss at tissue and cellular levels. J Bone Miner Res 5:311–319
Kim H-J (2010) New understanding of glucocorticoid action on bone cells. BMB Rep 43:524–529
Egerman M, Goldhahn J, Schneider E (2005) Animal models for fracture treatment in osteoporosis. Osteoporos Int 16:S 129–S 138
Peng Z, Tuukkanen I, Zhang H, Jamsa T, Vaananen HK (1994) The mechanical strength of bone in different rat models of experimental osteoporosis. Bone 15:523–532
Turner CH, Burr DB (1993) Basic biomechanical measurements of bone: a tutorial. Bone 14:595–608
Office of the Surgeon General (US) (2004) Bone health and osteoporosis: a report of the Surgeon General. Office of the Surgeon General (US), Rockville
Fávaro-Pípi E, Ribeiro DA, Ribeiro JU, Bossini P, Oliveira P, Parizotto NA, Tim C, de Araújo HS, Renno AC (2011) Low-level laser therapy induces differential expression of osteogenic genes during bone repair in rats. Photomed Laser Surg 29:311–317
Kiyosaki T, Mitsui N, Suzuki N, Shimizu N (2010) Low-level laser therapy stimulates mineralization via increased Runx2 expression and ERK phosphorylation in osteoblasts. Photomed Laser Surg 28(Suppl 1):S167–S172
Shimizu N, Mayahara K, Kiyosaki T, Yamaguchi A, Ozawa Y, Abiko Y (2007) Low-intensity laser irradiation stimulates bone nodule formation via insulin-like growth factor-I expression in rat calvarial cells. Lasers Surg Med 39:551–559
Luger EJ, Rochkind S, Wollman Y, Kogan G, Dekel S (1998) Effect of low power laser irradiation on the mechanical properties of bone fracture healing in rats. Lasers Surg Med 22:97–102
Trelles MA, Mayaio E (1987) Bone fracture consolidates faster with low-power laser. Lasers Surg Med 7:36–45
Pinheiro AL, Limeira Júnior Fde A, Gerbi ME, Ramalho LM, Marzola C, Ponzi EA, Soares AO, De Carvalho LC, Lima HC, Gonçalves TO (2003) Effect of 830-nm laser light on the repair of bone defects grafted with inorganic bovine bone and decalcified cortical osseus membrane. J Clin Laser Med Surg 21:301–306
Bossini PS, Rennó AC, Ribeiro DA, Fangel R, Ribeiro AC, Lahoz Mde A, Parizotto NA (2012) Low level laser therapy (830nm) improves bone repair in osteoporotic rats: similar outcomes at two different dosages. Exp Gerontol 47:136–142
Ko CY, Kang H, Ryu Y, Jung B, Kim H, Jeong D, Shin HI, Lim D, Kim HS (2013) The effects of minimally invasive laser needle system on suppression of trabecular bone loss induced by skeletal unloading. Lasers Med Sci 28:1495–1502
Ko CY, Kang H, Seo DH, Jung B, Schreiber J, Kim HS (2013) Low-level laser therapy using the minimally invasive laser needle system on osteoporotic bone in ovariectomized mice. Med Eng Phys 35:1015–1019
Renno AC, de Moura FM, dos Santos NS, Tirico RP, Bossini PS, Parizotto NA (2006) Effects of 830-nm laser light on preventing bone loss after ovariectomy. Photomed Laser Surg 24:642–645
Medalha CC, Amorim BO, Ferreira JM, Oliveira P, Pereira RM, Tim C, Lirani-Galvão AP, da Silva OL, Renno AC (2010) Comparison of the effects of electrical field stimulation and low-level laser therapy on bone loss in spinal cord-injured rats. Photomed Laser Surg 28:669–674
Muniz Renno AC, de Moura FM, dos Santos NS, Tirico RP, Bossini PS, Parizotto NA (2006) The effects of infrared-830 nm laser on exercised osteopenic rats. Lasers Med Sci 21:202–207
Ueda Y, Shimizu N (2001) Pulse irradiation of low-power laser stimulates bone nodule formation. J Oral Sci 43:55–60
Xu M, Deng T, Mo F, Deng B, Lam W, Deng P, Zhang X, Liu S (2009) Low-intensity pulsed laser irradiation affects RANKL and OPG mRNA expression in rat calvarial cells. Photomed Laser Surg 27:309–315
Ueda Y, Shimizu N (2003) Effects of pulse frequency of low-level laser therapy (LLLT) on bone nodule formation in rat calvarial cells. J Clin Laser Med Surg 21(5):271–277
Duan J, Na Y, Liu Y, Zhang Y (2012) Effects of the pulse frequency of low-level laser therapy on the tooth movement speed of rat molars. Photomed Laser Surg 30:663–667
Saad A, El Yamany M, Abbas O, Yehia M (2010) Possible role of low level laser therapy on bone turnover in ovariectomized rats. Endocr Regul 44:155–163
Javadieh F, Bayat M, Abdi S, Mohsenifar Z, Razi S (2009) The effects of infrared low-level laser therapy on healing of partial osteotomy of tibia in streptozotocin-induced diabetic rats. Photomed Laser Surg 27:641–646
Bayat M, Abdi S, Javadieh F, Mohsenifar Z, Rashid MR (2009) The effects of low-level laser therapy on bone in diabetic and non diabetic rats. Photomed Laser Surg 27:703–708
Li X, Ominsky MS, Warmington KS, Niu QT, Asuncion FJ, Barrero M, Dwyer D, Grisanti M, Stolina M, Kostenuik PJ, Simonet WS, Paszty C, Ke HZ (2011) Increased bone formation and bone mass induced by sclerostin antibody is not affected by pretreatment or cotreatment with alendronate in osteopenic, ovariectomized rats. Endocrinology 152:3312–3322
Ferretti JL, Gaffuri O, Capozza R, Cointry G, Bozzini C, Olivera M, Zanchetta JR, Bozzini CE (1995) Dexamethasone effects on mechanical, geometric and densitometric properties of rat femur diaphyses as described by peripheral quantitative computerized tomography and bending tests. Bone 16:119–124
Sun P, Cai DH, Li QN, Chen H, Deng WM, He L, Yang L (2010) Effects of alendronate and strontium ranelate on cancellous and cortical bone mass in glucocorticoid-treated adult rats. Calcif Tissue Int 86:495–501
Abdi S, Bayat M, Javadieh F, Mohsenifar Z, Rezaie F, Bayat M (2009) The effects of helium-neon light therapy on healing of partial osteotomy of the tibia in streptozotocin induced diabetic rats. Photomed Laser Surg 27:907–912
King CS, Weir EC, Gundberg CW, Fox J, Insogna KL (1996) Effects of continuous glucocorticoid infusion on bone metabolism in the rat. Calcif Tissue Int 59:184–191
Henneicke H, Hermann M, Kalak R et al (2011) Corticosterone selectively targets endo-cortical surfaces by an osteoblast-dependent mechanism. Bone 49:733–742
Mc Donough AK, Curtis JR, Saag KG (2008) The epidemiology of glucocorticoid-assotiated adverse events. Curr Opin Rheumatol 20:131–137
Diniz JS, Nicolau RA, de Melo Ocarino N, do Carmo Magalhaães F, de Oliveira Pereira RD, Serakides R (2009) Effect of low-power gallium-aluminum-arsenium laser therapy (830 nm) in combination with bisphosphonate treatment on osteopenic bone structure: an experimental animal study. Lasers Med Sci 24:347–352
Kamali F, Bayat M, Torkaman G, Ebrahimi E, Salavati M (2007) The therapeutic effect of low-level laser on repair of osteochondral defects in rabbit knee. The therapeutic effect of low-level laser on repair of osteochondral defects in rabbit knee. J Photochem Photobiol B 27(88):11–15
Einhorn TA (1992) Bone strength: the bottom line. Calcif Tissue Int 51:333–339
Mester E, Mester AF, Mester A (1985) The biomedical effects of laser application. Lasers Surg Med 5:31–39
Bayat M (2014) The necessity for increased attention to pulsed low-level laser therapy. Photomed Laser Surg 32:427–428
Ezzati A, Bayat M, Khoshvaghti A (2009) Low-level laser therapy with a pulsed infrared laser accelerates second-degree burn healing in rat: a clinical and microbiologic study. Photomed Laser Surg 28:603–611
Vasheghani MM, Bayat M, Dadpay M, Habibie M, Rezaei F (2009) Low-level laser therapy using 80-Hz pulsed infrared diode laser accelerates third-degree burn healing in rat. Photomed Laser Surg 27:959–964
Acknowledgments
We wish to extend our sincere thanks to the late Mrs. Jamileh Rezaei. We wish to express our appreciation to the Vice Chancellor of Research at Shahid Behesti University of Medical Sciences for financial support (grant no. 1392-1-115-1160).
Conflict of interest
No competing financial interests exist.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fridoni, M., Masteri Farahani, R., Nejati, H. et al. Evaluation of the effects of LLLT on biomechanical properties of tibial diaphysis in two rat models of experimental osteoporosis by a three point bending test. Lasers Med Sci 30, 1117–1125 (2015). https://doi.org/10.1007/s10103-014-1706-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-014-1706-1