Abstract
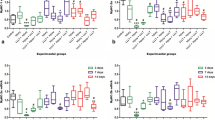
It has been demonstrated that reactive oxygen species (ROS) formation and oxidative damage markers are increased after muscle damage. Recent studies have demonstrated that low-level laser therapy (LLLT) modulates many biochemical processes mainly those related to reduction of muscular injures, increment of mitochondrial respiration and ATP synthesis, as well as acceleration of the healing process. The objective of the present investigation was to verify the influence of LLLT in some parameters of muscular injury, oxidative damage, antioxidant activity, and synthesis of collagen after traumatic muscular injury. Adult male Wistar rats were divided randomly into three groups (n = 6), namely, sham (uninjured muscle), muscle injury without treatment, and muscle injury with LLLT (GaAs, 904 nm). Each treated point received 5 J/cm2 or 0.5 J of energy density (12.5 s) and 2.5 J per treatment (five regions). LLLT was administered 2, 12, 24, 48, 72, 96, and 120 h after muscle trauma. The serum creatine kinase activity was used as an index of skeletal muscle injury. Superoxide anion, thiobarbituric acid reactive substance (TBARS) measurement, and superoxide dismutase (SOD) activity were used as indicators of oxidative stress. In order to assess the synthesis of collagen, levels of hydroxyproline were measured. Our results have shown that the model of traumatic injury induces a significant increase in serum creatine kinase activity, hydroxyproline content, superoxide anion production, TBARS level, and activity of SOD compared to control. LLLT accelerated the muscular healing by significantly decreasing superoxide anion production, TBARS levels, the activity of SOD, and hydroxyproline content. The data strongly indicate that increased ROS production and augmented collagen synthesis are elicited by traumatic muscular injury, effects that were significantly decreased by LLLT.
Similar content being viewed by others
References
Beiner JM, Jokl P (1997) The effect of anabolic steroids and corticosteroids on healing of muscle contusion injury. Am J Sports Med 27:2–9
Freitas TP, Gomes M, Fraga DB (2010) Effect of therapeutic pulsed ultrasound on lipoperoxidation and fibrogenesis in an animal model of wound healing. J Surg Res 161:168–171
Pattwell DM, Jackson MJ (2004) Contraction-induced oxidants as mediators of adaptation and damage in skeletal muscle. Exerc Sport Sci Rev 32:14–18
Elmali N, Esenkaya I, Karadag N et al (2007) Effects of resveratrol on skeletal muscle in ischemia-reperfusion injury. Ulus Travma Acil Cerrahi Derg 13:274–280
Schaser KD, Bail HJ, Schewior L (2005) Acute effects of N-acetylcysteine on skeletal muscle microcirculation following closed soft tissue trauma in rats. J Orthop Res 23:231–241
Bar-Shai M, Carmeli E, Ljubuncic P et al (2008) Exercise and immobilization in aging animals: the involvement of oxidative stress and NF-κB activation. Free Radic Biol Med 44:202–214
Halliwell B, Gutteridge JMC (2007) Free radical in biology medicine. Oxford University Press, New York
Ozyurt B, Iraz M, Koca K et al (2006) Protective effects of caffeic acid phenethyl ester on skeletal muscle ischemia-reperfusion injury in rats. Mol Cell Biochem 292:197–203
Shefer G, Barash I (2003) Low-energy laser irradiation enhances de novo protein synthesis via its effects on translation-regulatory proteins in skeletal muscle myoblasts. Biochim Biophys Acta 1593:131–139
Baptista J, Martins DM, Pavesi VCS (2011) Influence of laser photobiomodulation on collagen IV during skeletal muscle tissue remodeling after injury in rats. Photomed Laser Surg 29:11–17
Gao X, Xing D (2009) Molecular mechanisms of cell proliferation induced by low power laser irradiation. J Biomed Sci 16:4–20
Lakyova L, Toporcer T, Tomeckova V (2010) Low-level laser therapy for protection against skeletal muscle damage after ischemia–reperfusion injury in rat hindlimbs. Lasers Surg Med 42:665–672
Demir H, Yaray S (2004) Comparison of the effects of laser and ultrasound treatments on experimental wound healing in rats. J Rehabil Res Dev 41:721–728
Medrado A, Pugliese LS, Reis SR et al (2003) Influence of low level laser therapy on wound healing and its biological action upon myofibroblasts. Lasers Surg Med 32:239–244
Karu T (1999) Primary and secondary mechanisms of action of visible to near-IR radiation on cells. J Photochem Photobiol 49:1–17
Gulsoy M, Dereli Z (2006) Closure of skin incisions by 980-nm diode laser welding. Lasers Med Sci 21:5–10
Rizzi CF, Mauriz JL, Corrêa DSF et al (2006) Effects of low-level laser therapy (LLLT) on the nuclear factor (NF)-kB signaling pathway in traumatized muscle. Lasers Surg Med 38:704–713
Lopes-Martins RA, Marcos RL, Leonardo OS et al (2006) Effect of low-level laser (Ga-Al-As 655 nm) on skeletal muscle fatigue induced by electrical stimulation in rats. J Appl Physiol 101:283–288
Douris P, Southard V, Ferrigi R et al (2006) Effect of phototherapy on delayed onset muscle soreness. Photomed Laser Surg 24:377–382
Kelencz CA, Muñoz IS, Amorim CF, Nicolau RA (2010) Effect of low-power gallium-aluminum-arsenium noncoherent light (640 nm) on muscle activity: a clinical study. Photomed Laser Surg 28:647–652
de Almeida P, Lopes-Martins RÁ, Tomazoni SS (2011) Low-level laser therapy improves skeletal muscle performance, decreases skeletal muscle damage and modulates mRNA expression of COX-1 and COX-2 in a dose-dependent manner. Photochem Photobiol 87:1159–1163
Dias FJ, Issa JP, Vicentini FT et al (2011) Effects of low-level laser therapy on the oxidative metabolism and matrix proteins in the rat masseter muscle. Photomed Laser Surg 29:677–684
Silveira PCL, Silva LA, Fraga DB et al (2009) Evaluation of mitochondrial respiratory chain activity in muscle healing by low-level laser therapy. Photochem Photobiol 95:89–92
Silveira PCL, Silva LA, Tuon T et al (2009) Effects of low-level laser therapy on epidermal oxidative response induced by wound healing. Rev Bras Fisioter 13:281–287
Morrone G, Guzzardella GA, Orienti L et al (1998) Muscular trauma treated with a Ga-Al-As diode laser: in vivo experimental study. Lasers Med Sci 13:293–298
Freitas LS, Freitas TP, Silveira PC et al (2007) Effect of therapeutic pulsed ultrasound on parameters of oxidative stress in skeletal muscle after injury. Cell Biol Int 31:482–488
Oliver IT (1955) A spectrophotometric method for the determination of creatine phosphokinase and myokinase. Biochem J 61:116–122
Rosalki SB (1967) An improved procedure for serum creatine phosphokinase determination. J Lab Clin Med 69:696–705
Menegali BT, Nesi RT, Souza PS et al (2009) The effects of physical exercise on the cigarette smoke-induced pulmonary oxidative response. Pulm Pharmacol Ther 22:567–573
Poderoso JJ, Carreras MC, Cl L et al (1996) Nitric oxide inhibits electron transfer and increases superoxide radical production in rat heart mitochondrial and submitochondrial particles. Arch Biochem Biophes 328:85–92
Draper HH, Hadley M (1990) Malondialdehyde determination as index of lipid peroxidation. Meth Enzymol 186:421–431
Mccord JM, Fridovich I (1969) Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem 244:6049–6055
Lowry OH, Rosebough NG, Farr AL et al (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275
Amaral AC, Parizotto NA, Salvini TF et al (2001) Dose-dependency of low-energy HeNe laser effect in regeneration of skeletal muscle in mice. Lasers Med Sci 16:44–51
Carvalho PTC, Mazzer NRFA et al (2006) Analysis of the influence of low-power HeNe laser on the healing of skin wounds in diabetic and non-diabetic rats. Acta Cir Bras 21:177–183
Myllyharju J, Kivirikko KI (2001) Collagens and collagen-related diseases. Ann Med 33:7–21
Fillipin LI, Mauriz JL (2005) Low-level laser therapy (LLLT) prevents oxidative stress and reduces fibrosis in rat traumatized achilles tendon. Lasers Surg Med 37:293–300
Kerkweg U, Petrat F, Korth HG et al (2007) Disruption of skeletal myocytes initiates superoxide release: contribution of NAD(P)H oxidase. Shock 27:552–558
Supinski GS, Callahan LA (2007) Free radical-mediated skeletal muscle dysfunction in inflammatory conditions. J Appl Physiol 102:2056–2063
Karu TI, Pyatibrat LV, Kalendo GS (2004) Photobiological modulation of cell attachment via cytochrome C oxidase. Photochem Photobiol Sci 3:211–216
Tafur J, Mills PJ (2008) Low-intensity light therapy: exploring the role of redox mechanisms. Photomed Laser Surg 26:323–328
Karu T (2010) Mitochondrial mechanisms of photobiomodulation in context of new data about multiple roles of ATP. Photomed Laser Surg 28:159–160
Hamblin MR, Demidova TN (2006) Mechanisms of low level light therapy. SPIE 6140:01–12
Karu L, Pyatibrat G, Kalendo T (1995) Irradiation with He-Ne laser increases ATP level in cells cultivated in vitro. J Photochem Photobiol B 27:219–223
Yu W, Naim JO, Mcgowan M et al (1997) Photomodulation of oxidative metabolism and electron chain enzymes in rat liver mitochondria. Photochem Photobiol 66:866–871
Karu T (1987) Photobiological fundamentals of low-power laser therapy. J Quantum Electron 23:1703–1717
Ghamsari SM, Taguchi K, Abe N et al (1997) Evaluation of low level laser therapy on primary healing of experimentally induced full thickness teat wounds in dairy cattle. Vet Surg 26:114–120
Yamaya M, Shiroto C, Kobayashi H et al (1993) Mechanistic approach to GaAIAs diode laser effects on production of reactive oxygen species from human neutrophils as a model for therapeutic modality at cellular level. Laser Ther 5:111–116
Avni D, Levkovitz S, Maltz L et al (2005) Protection of skeletal muscles from ischemic injury: low-level laser therapy increases antioxidant activity. Photomed Laser Surg 23:273–277
Kim YG, Pak SC, Lee SR et al (2000) Hairless mouse epidermal antioxidants and lipid peroxidation assessed by He-Ne laser. Lasers Surg Med 27:420–426
Dourado DM, Fávero S, Matias R et al (2011) Low-level laser therapy promotes vascular endothelial growth factor receptor-1 expression in endothelial and nonendothelial cells of mice gastrocnemius exposed to snake venom. Photochem Photobiol 87:418–426
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Silveira, P.C.L., da Silva, L.A., Pinho, C.A. et al. Effects of low-level laser therapy (GaAs) in an animal model of muscular damage induced by trauma. Lasers Med Sci 28, 431–436 (2013). https://doi.org/10.1007/s10103-012-1075-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-012-1075-6