Abstract
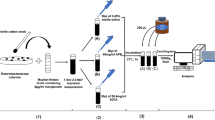
Plasmid-mediated quinolone resistance mechanisms have become increasingly prevalent among Enterobacteriaceae strains since the 1990s. Among these mechanisms, AAC(6’)-Ib-cr is the most difficult to detect. Different detection methods have been developed, but they require expensive procedures such as Sanger sequencing, pyrosequencing, polymerase chain reaction (PCR) restriction, or the time-consuming phenotypic method of Wachino. In this study, we describe a simple matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) method which can be easily implemented in clinical laboratories that use the MALDI-TOF technique for bacterial identification. We tested 113 strains of Enterobacteriaceae, of which 64 harbored the aac(6’)-Ib-cr gene. We compared two MALDI-TOF strategies, which differed by their norfloxacin concentration (0.03 vs. 0.5 g/L), and the method of Wachino with the PCR and sequencing strategy used as the reference. The MALDI-TOF strategy, performed with 0.03 g/L norfloxacin, and the method of Wachino yielded the same high performances (Se = 98 %, Sp = 100 %), but the turnaround time of the MALDI-TOF strategy was faster (<5 h), simpler, and inexpensive (<1 Euro). Our study shows that the MALDI-TOF strategy has the potential to become a major method for the detection of many different enzymatic resistance mechanisms.


Similar content being viewed by others
References
Strahilevitz J, Jacoby GA, Hooper DC, Robicsek A (2009) Plasmid-mediated quinolone resistance: a multifaceted threat. Clin Microbiol Rev 22:664–689. doi:10.1128/CMR.00016-09
Robicsek A, Strahilevitz J, Sahm DF, Jacoby GA, Hooper DC (2006) qnr prevalence in ceftazidime-resistant Enterobacteriaceae isolates from the United States. Antimicrob Agents Chemother 50:2872–2874. doi:10.1128/AAC.01647-05
Robicsek A, Strahilevitz J, Jacoby GA, Macielag M, Abbanat D, Park CH et al (2006) Fluoroquinolone-modifying enzyme: a new adaptation of a common aminoglycoside acetyltransferase. Nat Med 12:83–88. doi:10.1038/nm1347
Guillard T, Duval V, Moret H, Brasme L, Vernet-Garnier V, de Champs C (2010) Rapid detection of aac(6’)-Ib-cr quinolone resistance gene by pyrosequencing. J Clin Microbiol 48:286–289. doi:10.1128/JCM.01498-09
Guillard T, Fontaine N, Limelette A, Lebreil A-L, Madoux J, de Champs C (2013) A simplified and cost-effective method combining real-time PCR and pyrosequencing for detection of aac(6’)-Ib-cr gene. J Microbiol Methods 95:268–271. doi:10.1016/j.mimet.2013.09.015
Hidalgo-Grass C, Strahilevitz J (2010) High-resolution melt curve analysis for identification of single nucleotide mutations in the quinolone resistance gene aac(6’)-Ib-cr. Antimicrob Agents Chemother 54:3509–3511. doi:10.1128/AAC.00485-10
Park CH, Robicsek A, Jacoby GA, Sahm D, Hooper DC (2006) Prevalence in the United States of aac(6’)-Ib-cr encoding a ciprofloxacin-modifying enzyme. Antimicrob Agents Chemother 50:3953–3955. doi:10.1128/AAC.00915-06
Wachino J-I, Yamane K, Arakawa Y (2011) Practical disk-based method for detection of Escherichia coli clinical isolates producing the fluoroquinolone-modifying enzyme AAC(6’)-Ib-cr. J Clin Microbiol 49:2378–2379. doi:10.1128/JCM.00278-11
Hrabák J (2015) Detection of carbapenemases using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) meropenem hydrolysis assay. Methods Mol Biol 1237:91–96. doi:10.1007/978-1-4939-1776-1_9
Comité de l’Antibiogramme de la Société Française de Microbiologie (CA-SFM) (2013) Recommandations
Parshikov IA, Heinze TM, Moody JD, Freeman JP, Williams AJ, Sutherland JB (2001) The fungus Pestalotiopsis guepini as a model for biotransformation of ciprofloxacin and norfloxacin. Appl Microbiol Biotechnol 56:474–477
Dubois D, Grare M, Prere M-F, Segonds C, Marty N, Oswald E (2012) Performances of the Vitek MS matrix-assisted laser desorption ionization-time of flight mass spectrometry system for rapid identification of bacteria in routine clinical microbiology. J Clin Microbiol 50:2568–2576. doi:10.1128/JCM.00343-12
Harris P, Winney I, Ashhurst-Smith C, O’Brien M, Graves S (2012) Comparison of Vitek MS (MALDI-TOF) to standard routine identification methods: an advance but no panacea. Pathology 44:583–555. doi:10.1097/PAT.0b013e328358343c
Burckhardt I, Zimmermann S (2011) Using matrix-assisted laser desorption ionization-time of flight mass spectrometry to detect carbapenem resistance within 1 to 2.5 hours. J Clin Microbiol 49:3321–3324. doi:10.1128/JCM.00287-11
Hrabák J, Studentová V, Walková R, Zemlicková H, Jakubu V, Chudácková E et al (2012) Detection of NDM-1, VIM-1, KPC, OXA-48, and OXA-162 carbapenemases by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J Clin Microbiol 50:2441–2443. doi:10.1128/JCM.01002-12
Acknowledgments
We thank Laurent Guillouard and Alexis Pontvianne for their technical assistance. This work was presented in part at the 31ème Réunion Interdisciplinaire de Chimiothérapie Anti-Infectieuse (RICAI), Paris, France, December 2011.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding sources
This work was supported by a grant from the French Ministry of Health via the Institut de Veille Sanitaire and by a grant from the Centre Hospitalier Régional Universitaire de Clermont-Ferrand, France
Conflict of interest
All authors report no conflicts of interest in relation to this article.
Rights and permissions
About this article
Cite this article
Pardo, CA., Tan, R.N., Hennequin, C. et al. Rapid detection of AAC(6’)-Ib-cr production using a MALDI-TOF MS strategy. Eur J Clin Microbiol Infect Dis 35, 2047–2051 (2016). https://doi.org/10.1007/s10096-016-2762-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-016-2762-1