Abstract
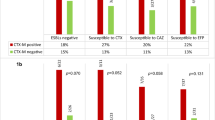
The aim of the study was to investigate the epidemiology and clinical features of bloodstream infections due to Escherichia coli producing AmpC β-lactamases (AmpC-Ec-BSI). In a multi-centre case–control study, all third-generation-cephalosporin-resistant Escherichia coli BSI (3GC-Ec-BSI) isolates were analysed. Acquired bla AmpC (bla ac-AmpC) detection was done by polymerase chain reaction (PCR) and sequencing. Chromosomal bla AmpC (bla c-AmpC) expression was quantified by real-time PCR. Cases were patients with AmpC-Ec-BSI. Controls were patients with cephalosporin-susceptible E. coli BSI, matched 1:1 by sex and age. Demographics, comorbidities, intrinsic and extrinsic risk factors for antimicrobial resistance, clinical presentation and outcomes were investigated. Among 841 E. coli BSI, 17 were caused by AmpC-Ec (2 %). Eleven isolates (58.8 %) had bla ac-AmpC and six were bla c-AmpC overproducers. The mean age of cases was 66.2 years and 71 % were men. Cases were more frequently healthcare-related (82 vs. 52 % controls, p < 0.05) and presented more intrinsic and extrinsic risk factors. At least one risk factor was present in 94.1 % of cases vs. 41.7 % of controls (p = 0.002). Severity and length of stay (LOS) were higher among cases (mean Pitt Score 2.6 vs. 0.38 in controls, p = 0.03; LOS 17.5 days vs. 6 in controls, p = 0.02). Inappropriate empirical therapy (IET) was administered to 70.6 % of cases and 23.5 % of controls (p < 0.003). No differences were found in terms of cure rate at the 14th day and mortality. Bloodstream infections due to AmpC-Ec (mostly plasmid-mediated) are infrequent in our area. AmpC-Ec-BSI affects mainly patients with intrinsic risk factors and those with previous antibiotic exposure. A high proportion received IET.
Similar content being viewed by others
References
Martin GS, Mannino DM, Eaton S, Moss M (2003) The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med 348:1546–1554
Wilson J, Elgohari S, Livermore DM, Cookson B, Johnson A, Lamagni T et al (2011) Trends among pathogens reported as causing bacteraemia in England, 2004–2008. Clin Microbiol Infect 17:451–458
de Kraker ME, Jarlier V, Monen JC, Heuer OE, van de Sande N, Grundmann H (2013) The changing epidemiology of bacteraemias in Europe: trends from the European Antimicrobial Resistance Surveillance System. Clin Microbiol Infect 19:860–868
Ammerlaan HS, Harbarth S, Buiting AG, Crook DW, Fitzpatrick F, Hanberger H et al (2013) Secular trends in nosocomial bloodstream infections: antibiotic-resistant bacteria increase the total burden of infection. Clin Infect Dis 56:798–805
European Centre for Disease Prevention and Control. Antimicrobial resistance surveillance in Europe 2009. Annual Report of the European Antimicrobial Resistance Surveillance Network (EARS-Net). Stockholm: ECDC; 2010. Available online at: http://ecdc.europa.eu/en/publications/Publications/1011_SUR_annual_EARS_Net_2009.pdf
Jacoby GA (2009) AmpC beta-lactamases. Clin Microbiol Rev 22:161–182
Hawkey PM, Jones AM (2009) The changing epidemiology of resistance. J Antimicrob Chemother 64(Suppl 1):i3–i10
Miró E, Agüero J, Larrosa MN, Fernández A, Conejo MC, Bou G et al (2013) Prevalence and molecular epidemiology of acquired AmpC β-lactamases and carbapenemases in Enterobacteriaceae isolates from 35 hospitals in Spain. Eur J Clin Microbiol Infect Dis 32:253–259
Denisuik AJ, Lagacé-Wiens PR, Pitout JD, Mulvey MR, Simner PJ, Tailor F et al (2013) Molecular epidemiology of extended-spectrum β-lactamase-, AmpC β-lactamase- and carbapenemase-producing Escherichia coli and Klebsiella pneumoniae isolated from Canadian hospitals over a 5 year period: CANWARD 2007-11. J Antimicrob Chemother 68(Suppl 1):i57–i65
Pascual V, Ortiz G, Simó M, Alonso N, Garcia MC, Xercavins M et al (2015) Epidemiology and risk factors for infections due to AmpC β-lactamase-producing Escherichia coli. J Antimicrob Chemother 70:899–904
Pallett A, Hand K (2010) Complicated urinary tract infections: practical solutions for the treatment of multiresistant Gram-negative bacteria. J Antimicrob Chemother 65(Suppl 3):iii25–iii33
Livermore DM, Canton R, Gniadkowski M, Nordmann P, Rossolini GM, Arlet G et al (2007) CTX-M: changing the face of ESBLs in Europe. J Antimicrob Chemother 59:165–174
Mammeri H, Nordmann P, Berkani A, Eb F (2008) Contribution of extended-spectrum AmpC (ESAC) beta-lactamases to carbapenem resistance in Escherichia coli. FEMS Microbiol Lett 282:238–240
Martínez-Martínez L, Pascual A, Hernández-Allés S, Alvarez-Díaz D, Suárez AI, Tran J et al (1999) Roles of beta-lactamases and porins in activities of carbapenems and cephalosporins against Klebsiella pneumoniae. Antimicrob Agents Chemother 43:1669–1673
Charlson ME, Pompei P, Ales KL, MacKenzie CR (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40:373–383
Friedman ND, Kaye KS, Stout JE, McGarry SA, Trivette SL, Briggs JP et al (2002) Health care-associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann Intern Med 137:791–797
Clinical and Laboratory Standards Institute (CLSI) (2010) Performance standards for antimicrobial susceptibility testing: Twentieth informational supplement. CLSI document M100-S20. CLSI, Wayne, PA, USA
Bauer AW, Kirby WM, Sherris JC, Turck M (1966) Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol 45:493–496
Navarro F, Calvo J, Cantón R, Fernández-Cuenca F, Mirelis B (2011) Deteccion fenotipica de mecanismos de resistencia en microorganismos gramnegativos. Enferm Infecc Microbiol Clin 29:524–534
Mirelis B, Rivera A, Miró E, Mesa RJ, Navarro F, Coll P (2006) A simple phenotypic method for differentiation between acquired and chromosomal AmpC β-lactamases in Escherichia coli. Enferm Infecc Microbiol Clin 24:370–372
Pérez-Pérez FJ, Hanson ND (2002) Detection of plasmid-mediated AmpC β-lactamase genes in clinical isolates by using multiplex PCR. J Clin Microbiol 40:2153–2162
Alonso N, Miró E, Pascual V, Rivera A, Simó M, Garcia MC et al (2016) Molecular characterisation of acquired and overproduced chromosomal bla AmpC in Escherichia coli clinical isolates. Int J Antimicrob Agents 47:62–68
Alvarez M, Tran JH, Chow N, Jacoby GA (2004) Epidemiology of conjugative plasmid-mediated AmpC beta-lactamases in the United States. Antimicrob Agents Chemother 48:533–537
Courpon-Claudinon A, Lefort A, Panhard X, Clermont O, Dornic Q, Fantin B et al (2011) Bacteraemia caused by third-generation cephalosporin-resistant Escherichia coli in France: prevalence, molecular epidemiology and clinical features. Clin Microbiol Infect 17:557–565
Gude MJ, Seral C, Sáenz Y, Cebollada R, González-Domínguez M, Torres C et al (2013) Molecular epidemiology, resistance profiles and clinical features in clinical plasmid-mediated AmpC-producing Enterobacteriaceae. Int J Med Microbiol 303:553–557
Matsumura Y, Nagao M, Iguchi M, Yagi T, Komori T, Fujita N et al (2013) Molecular and clinical characterization of plasmid-mediated AmpC β-lactamase-producing Escherichia coli bacteraemia: a comparison with extended-spectrum β-lactamase-producing and non-resistant E. coli bacteraemia. Clin Microbiol Infect 19:161–168
Matsumura Y, Yamamoto M, Higuchi T, Komori T, Tsuboi F, Hayashi A et al (2012) Prevalence of plasmid-mediated AmpC β-lactamase-producing Escherichia coli and spread of the ST131 clone among extended-spectrum β-lactamase-producing E. coli in Japan. Int J Antimicrob Agents 40:158–162
Datta S, Wattal C, Goel N, Oberoi JK, Raveendran R, Prasad KJ (2012) A ten year analysis of multi-drug resistant blood stream infections caused by Escherichia coli & Klebsiella pneumoniae in a tertiary care hospital. Indian J Med Res 135:907–912
Yamasaki K, Komatsu M, Abe N, Fukuda S, Miyamoto Y, Higuchi T et al (2010) Laboratory surveillance for prospective plasmid-mediated AmpC beta-lactamases in the Kinki region of Japan. J Clin Microbiol 48:3267–3273
Pai H, Kang CI, Byeon JH, Lee KD, Park WB, Kim HB et al (2004) Epidemiology and clinical features of bloodstream infections caused by AmpC-type-β-lactamase-producing Klebsiella pneumoniae. Antimicrob Agents Chemother 48:3720–3728
Calbo E, Romaní V, Xercavins M, Gómez L, Vidal CG, Quintana S et al (2006) Risk factors for community-onset urinary tract infections due to Escherichia coli harbouring extended-spectrum beta-lactamases. J Antimicrob Chemother 57:780–783
Ben-Ami R, Rodríguez-Baño J, Arslan H, Pitout JD, Quentin C, Calbo ES et al (2009) A multinational survey of risk factors for infection with extended-spectrum beta-lactamase-producing enterobacteriaceae in nonhospitalized patients. Clin Infect Dis 49:682–690
Picard B, Garcia JS, Gouriou S, Duriez P, Brahimi N, Bingen E et al (1999) The link between phylogeny and virulence in Escherichia coli extraintestinal infection. Infect Immun 67:546–553
Rodríguez-Baño J, Oteo J, Ortega A, Villar M, Conejo MC, Bou G et al (2013) Epidemiological and clinical complexity of amoxicillin-clavulanate-resistant Escherichia coli. J Clin Microbiol 51:2414–2417
Lee CH, Su LH, Li CC, Chien CC, Tang YF, Liu JW (2010) Microbiologic and clinical implications of bacteremia due to extended-spectrum-beta-lactamase-producing Klebsiella pneumoniae with or without plasmid-mediated AmpC beta-lactamase DHA-1. Antimicrob Agents Chemother 54:5395–5398
Corvec S, Crémet L, Leprince C, Dauvergne S, Reynaud A, Lepelletier D et al (2010) Epidemiology of Escherichia coli clinical isolates producing AmpC plasmidic beta-lactamase during a 5-year period in a French teaching hospital. Diagn Microbiol Infect Dis 67:277–281
Jørgensen RL, Nielsen JB, Friis-Møller A, Fjeldsøe-Nielsen H, Schønning K (2010) Prevalence and molecular characterization of clinical isolates of Escherichia coli expressing an AmpC phenotype. J Antimicrob Chemother 65:460–464
Naseer U, Haldorsen B, Simonsen GS, Sundsfjord A (2010) Sporadic occurrence of CMY-2-producing multidrug-resistant Escherichia coli of ST-complexes 38 and 448, and ST131 in Norway. Clin Microbiol Infect 16:171–178
Oteo J, Cercenado E, Cuevas O, Bautista V, Delgado-Iribarren A, Orden B et al (2010) AmpC beta-lactamases in Escherichia coli: emergence of CMY-2-producing virulent phylogroup D isolates belonging mainly to STs 57, 115, 354, 393, and 420, and phylogroup B2 isolates belonging to the international clone O25b-ST131. Diagn Microbiol Infect Dis 67:270–276
Bukh AS, Schønheyder HC, Emmersen JM, Søgaard M, Bastholm S, Roslev P (2009) Escherichia coli phylogenetic groups are associated with site of infection and level of antibiotic resistance in community-acquired bacteraemia: a 10 year population-based study in Denmark. J Antimicrob Chemother 64:163–168
Cooke NM, Smith SG, Kelleher M, Rogers TR (2010) Major differences exist in frequencies of virulence factors and multidrug resistance between community and nosocomial Escherichia coli bloodstream isolates. J Clin Microbiol 48:1099–1104
Rodríguez-Baño J, Mingorance J, Fernández-Romero N, Serrano L, López-Cerero L, Pascual A et al (2012) Virulence profiles of bacteremic extended-spectrum β-lactamase-producing Escherichia coli: association with epidemiological and clinical features. PLoS One 7, e44238
Tam VH, Chang KT, Schilling AN, LaRocco MT, Genty LO, Garey KW (2009) Impact of AmpC overexpression on outcomes of patients with Pseudomonas aeruginosa bacteremia. Diagn Microbiol Infect Dis 63:279–285
Paltansing S, Kraakman ME, Ras JM, Wessels E, Bernards AT et al (2013) Characterization of fluoroquinolone and cephalosporin resistance mechanisms in Enterobacteriaceae isolated in a Dutch teaching hospital reveals the presence of an Escherichia coli ST131 clone with a specific mutation in parE. J Antimicrob Chemother 68:40–45
Robicsek A, Jacoby GA, Hooper DC (2006) The worldwide emergence of plasmid-mediated quinolone resistance. Lancet Infect Dis 6:629–640
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
J. G. has accepted grants from Vifor Pharma, Bayer and Pfizer, and speaking engagements and conference invitations from Astellas, AstraZeneca, Novartis, Pfizer, GSK, Bayer, Vifor Pharma, Cubist, Durata and Theravance. E. C. has accepted grants, speaking engagements and conference invitations from Astellas, AstraZeneca, Novartis, Pfizer and MSD. All other authors: none to declare.
Informed consent and ethical approval
The study was approved by the Ethics Committee of the participating hospitals. Informed consent was not deemed necessary due to the retrospective observational design of the study.
Funding
This work was supported by the Fundació Mútua de Terrassa, Grant SQ1Number BFMT 2010/B and by REIPI, RD06 by Plan Nacional I+D+I 2008–2011 and Instituto de Salud Carlos III, Subdirección General de Redes y Centros de Investigación Cooperativa, Ministerio de Economía y Competitividad, Spanish Network for Research in Infectious Diseases (REIPI RD12/0015)—co-financed by European Development Regional Fund ‘A way to achieve Europe’ ERDF.
Rights and permissions
About this article
Cite this article
Pascual, V., Alonso, N., Simó, M. et al. Bloodstream infections caused by Escherichia coli producing AmpC β-lactamases: epidemiology and clinical features. Eur J Clin Microbiol Infect Dis 35, 1997–2003 (2016). https://doi.org/10.1007/s10096-016-2752-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-016-2752-3