Abstract
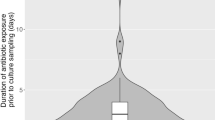
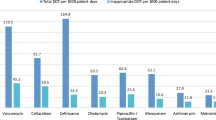
International - predominantly American - studies undertaken in the ICUs of teaching centres show that inadequate antibiotic therapy increases mortality and length of stay. We sought to ascertain whether this also pertains to smaller ICUs in the Veneto region of north-east Italy. To the best of our knowledge, this is the first such survey in the Veneto area or in Italy as a whole. A retrospective, observational study was performed across five general-hospital ICUs to examine appropriateness of microbiological sampling, empirical antibiotic adequacy, and outcomes. Among 911 patients (mean age, 65.8 years ± 16.2 SD; median ICU stay, 17.0 days [IQR, 8.0–29.0]), 757 (83.1 %) were given empirical antibiotics. Treatment adequacy could be fully assessed in only 212 patients (28.0 %), who received empirical treatment and who had a relevant clinical sample collected at the initiation of this antibiotic (T0). Many other patients only had delayed microbiological investigation of their infections between day 1 and day 10 of therapy. Mortality was significantly higher among the 34.9 % of patients receiving inadequate treatment (48.6 % vs 18.80 %; p < 0.001). Only 32.5 % of combination regimens comprised a broad-spectrum Gram-negative β-lactam plus an anti-MRSA agent, and many combinations were irrational. Inadequate treatment was frequent and was strongly associated with mortality; moreover, there was delayed microbiological investigation of many infections, precluding appropriate treatment modification and de-escalation. Improvements in these aspects and in antibiotic stewardship are being sought.


Similar content being viewed by others
References
Kollef M (2008) SMART approaches for reducing nosocomial infections in the ICU. Chest 134:447–456
Joram N, de Saint Blanquat L, Stamm D, Launay E, Gras-Le Guen C (2012) Healthcare-associated infection prevention in pediatric intensive care units: a review. Eur J Clin Microbiol Infect Dis 31:2481–2490
Osman MF, Askari R (2014) Infection control in the intensive care unit. Surg Clin North Am 94:1175–1194
Cohen ML (1992) Epidemiology of drug resistance: implications for a post-antimicrobial era. Science 257:1030–1055
Goldmann DA, Weinstein RA, Wenzel RP, Tablan OC, Duma RJ, Gaynes RP et al (1996) Strategies to prevent and control the emergence and spread of antibiotic-resistant organisms in hospitals - a challenge to hospital leadership. JAMA 275:234–240
Carbon C (1999) Costs of treating infections caused by methicillin-resistant staphylococci and vancomycin-resistant enterococci. J Antimicrob Chemother 44:31–36
Vincent JL (2000) Microbial resistance: lessons from the EPIC study. Intensive Care Med 26 [Suppl 1]:S3–S8
Vincent JL, Sakr Y, Sprung CL, Ranieri VM, Reinhart K, Gerlach H et al (2006) Sepsis in European intensive care units: results of the SOAP study. Crit Care Med 34:344–353
Raymond DP, Pelletier SJ, Crabtree DT, Evans HL, Pruett TL, Sawyer RG (2003) Impact of antibiotic-resistant Gram-negative bacilli infections on outcome in hospitalized patients. Crit Care Med 31:1035–1041
Cosgrove SE (2006) The relationship between antimicrobial resistance and patient outcomes: mortality, length of hospital stay, and health care costs. Clin Infect Dis 42 [Suppl 2]:82–89
Ammerlaan H, Seifert H, Harbarth S, Brun-Buisson C, Torres A, Antonelli M et al (2009) Adequacy of antimicrobial treatment and outcome of Staphylococcus aureus bacteremia in 9 Western European Countries. Clin Infect Dis 49:997–1005
Paruk F, Richards G, Scribante J, Bhagwanjee S, Mer M, Perrie H et al (2012) Antibiotic prescription practices and their relationship to outcome in South Africa: findings of the prevalence of infection in South African intensive care units (PISA) study. S Afr Med J 102:613–618
Kollef MH, Sherman G, Ward S, Fraser VJ (1999) Inadequate antimicrobial treatment of infections: a risk factor for hospital mortality among critically ill patients. Chest 115:462–474
Leibovici L, Shraga I, Drucker M, Konigsberger H, Samra Z, Pitlik SD (1998) The benefit of appropriate empirical antibiotic treatment in patients with bloodstream infection. J Intern Med 244:379–386
Ibrahim EH, Sherman G, Ward S, Fraser VJ, Kollef MH (2000) The influence of inadequate antimicrobial treatment of bloodstream infections on patient outcomes in the ICU setting. Chest 118:146–155
Vallés J, Rello J, Ochagavía A, Garnacho J, Alcalá MA (2003) Community-acquired bloodstream infection in critically ill adult patients. Impact of shock and inappropriate antibiotic therapy on survival. Chest 123:1615–1624
Cosgrove SE, Qi Y, Kaye KS, Harbarth S, Karchmer AW, Carmeli Y et al (2005) The impact of methicillin resistance in Staphylococcus aureus bacteremia on patient outcomes: mortality, length of stay, and hospital charges. Infect Control Hosp Epidemiol 26:166–174
Garnacho-Montero J, Ortiz-Leyba C, Herrera-Melero I, Aldabó-Pallás T, Cayuela-Dominguez A, Marquez-Vacaro JA et al (2008) Mortality and morbidity attributable to inadequate empirical antimicrobial therapy in patients admitted to the ICU with sepsis: a matched cohort study. J Antimicrob Chemother 61:436–441
Retamar P, Portillo MM, López-Prieto MD, Rodríguez-López F, de Cueto M, García MV et al (2012) Impact of inadequate empirical therapy on the mortality of patients with bloodstream infections: a propensity score-based analysis. Antimicrob Agents Chemother 56:472–478
Girometti N, Lewis RE, Giannella M, Ambretti S, Bartoletti M, Tedeschi S, Tumietto F, Cristini F, Trapani F, Gaibani P, Viale P (2014) Klebsiella pneumoniae bloodstream infection: epidemiology and impact of inappropriate empirical therapy. Medicine (Baltimore) 93:298–309
Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, Sharma S et al (2006) Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med 34:1589–1596
Carmeli Y, Troillet N, Karchmer AW, Samore MH (1999) Health and economic outcomes of antibiotic resistance in Pseudomonas aeruginosa. Arch Intern Med 159:1127–1132
Moroney JF, Fiore AE, Harrison LH, Patterson JE, Farley MM, Jorgensen JH et al (2001) Clinical outcomes of bacteremic pneumococcal pneumonia in the era of antibiotic resistance. Clin Infect Dis 33:797–805
Lautenbach E, Patel JB, Bilker WB, Edelstein PH, Fishman NO (2001) Extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae: risk factors for infection and impact of resistance on outcomes. Clin Infect Dis 32:1162–1171
Shorr AF (2009) Review of studies of the impact on Gram-negative bacterial resistance on outcomes in the intensive care unit. Crit Care Med 37:1463–1469
Shorr AF, Micek ST, Welch EC, Doherty JA, Reichley RM, Kollef MH (2011) Inappropriate antibiotic therapy in Gram-negative sepsis increases hospital length of stay. Crit Care Med 39:46–51
Italian Association of Hospital Anaesthetists. National Census of ICU beds, as of June 30th 2005. www.aaroi.it
World Health Organization. International Statistical Classification of Diseases and Related Health Problems—10th revision. http://apps.who.int/classifications/apps/icd/icd10online/
Kollef MH (2000) Inadequate antimicrobial treatment: an important determinant of outcome for hospitalized patients. Clin Infect Dis 31 [Suppl 4]:131–138
Cosgrove SE, Carmeli Y (2003) The impact of antimicrobial resistance on health and economic outcomes. Clin Infect Dis 36:1433–1437
World Medical Association. Declaration of Helsinki. Ethical principles for medical research involving human subjects. Adopted by the 18th WMA General Assembly, Helsinki, Finland, June 1964, and amended by the 55th WMA General Assembly, Tokyo 2004 www.wma.net/en/30publications/10policies/b3/17c.pdf
Barrett J, Edgeworth J, Wyncoll D (2015) Shortening the course of antibiotic treatment in the intensive care unit. Expert Rev Anti-Infect Ther 13:463–471. doi:10.1586/14787210.2015.1008451
Paterson DL, Ko WC, Von Gottberg A, Mohapatra S, Casellas JM, Goossens H et al (2004) Antibiotic therapy for Klebsiella pneumoniae bacteremia: implications of production of extended-spectrum β-lactamases. Clin Infect Dis 39:31–37
Tumbarello M, Sanguinetti M, Montuori E, Trecarichi EM, Posteraro B, Fiori B et al (2007) Predictors of mortality in patients with bloodstream infections caused by extended-spectrum-β-lactamase-producing Enterobacteriaceae: importance of inadequate initial antimicrobial treatment. Antimicrob Agents Chemother 51:1987–1994
Kumar A, Ellis P, Arabi Y, Roberts D, Light B, Parrillo JE et al (2009) Initiation of inappropriate antimicrobial therapy results in a fivefold reduction of survival in human septic shock. Chest 136:1237–1248
Siempos II, Vardakas KZ, Kyriakopoulos CE, Ntaidou TK, Falagas ME (2010) Predictors of mortality in adult patients with ventilator-associated pneumonia: a meta-analysis. Shock 33:590–601
Malacarne P, Rossi P, Bertolini G (2004) Antibiotic usage in intensive care units: a pharmaco-epidemiological multicentre study. J Antimicrob Chemother 54:221–224
Vaccheri A, Silvani MC, Bersaglia L, Motola D, Strahinja P, Vargiu A et al (2008) A 3 year survey on the use of antibacterial agents in five Italian hospitals. J Antimicrob Chemother 61:953–958
Vlahovic-Palcevski V, Francetic I, Palcevski G, Novak S, Abram M, Bergman U (2007) Antimicrobial use at a university hospital: appropriate or misused? A qualitative study. Int J Clin Pharmacol Ther 45:169–174
Kariv G, Paul M, Shani V, Muchtar E, Leibovici L (2013) Benchmarking inappropriate empirical antibiotic treatment. Clin Microbiol Infect 19:629–633
Nordmann P (2014) Carbapenemase-producing Enterobacteriaceae: overview of a major public health challenge. Med Mal Infect 44:51–56. doi:10.1016/j.medmal.2013.11.007
Corcione S, Rocchetti A, Argentero PA, Raso R, Zotti CM, De Rosa FG, Ghisetti V (2015) A one-year survey of carbapenemase-producing Klebsiella pneumoniae in Italy: beyond the ICU. Clin Microbiol Infect 21:e11–e13. doi:10.1016/j.cmi.2014.09.012
Albiger B, Glasner C, Struelens M, Grundmann H, Monnet D, European Survey of Carbapenemase-Producing Enterobacteriaceae (EuSCAPE) working group (2015) Carbapenemase-producing Enterobacteriaceae in Europe: assessment by national experts from 38 countries. Euro Surveill 20(45)30062. doi: 10.2807/1560-7917.ES.2015.20.45.30062
Acknowledgements
We are indebted to the Institutional Sanitary Boards of the participating hospitals for assistance with performing the study. We are also grateful to Dr P. Piccinni, Dr D. Mastropasqua, Dr M. Baiocchi, Dr G. Zanardo and Dr L. Ongaro for giving permission to examine patients’ clinical records in the participating ICUs. Special thanks to Prof A. Holmes and to Prof P. Wilson for their careful reading of the PhD thesis upon which this manuscript is based.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding of any kind has been received for this work, and data have been generated as part of the routine work. No financial support is ongoing for any of the authors. The manuscript has been written without any external professional support.
Conflicts of interest
David M. Livermore has performed on advisory boards or ad-hoc consultancy for Accelerate, Achaogen, Adenium, Allecra, AstraZeneca, Auspherix, Basilea, BioVersys, Centauri, Cubist, Cycle, Discuva, Meiji, Nordic, Pfizer, Roche, Shionogi, Tetraphase, VenatoRx, Wockhardt; paid lectures for AOP Orphan, AstraZeneca, Merck, Nordic, Pfizer; relevant shareholdings in Dechra, GSK, Merck, Perkin Elmer, Pfizer amounting to <10 % of portfolio value; contract research for Achaogen, Allecra Antiinfectives, AstraZeneca, Cubist Pharmaceuticals, GlaxoSmithKline, Merck, Meiji Melinta, and Wockhardt Ltd.
All the other authors declare that they have no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
For this type of study formal consent is not required. Please see also: Patients and methods.
Rights and permissions
About this article
Cite this article
Benedetti, P., Sefton, A.M., Menegozzo, M. et al. Antimicrobial use and microbiological testing in district general hospital ICUs of the Veneto region of north-east Italy. Eur J Clin Microbiol Infect Dis 35, 1627–1638 (2016). https://doi.org/10.1007/s10096-016-2701-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-016-2701-1