Abstract
In the present study, we challenged the concept that levofloxacin should not be used for the management of ventilator-associated pneumonia (VAP) when minimum inhibitory concentrations (MICs) exceed 2 μg/ml. Multidrug-resistant (MDR) and genetically distinct isolates of Pseudomonas aeruginosa (n = 49) and Acinetobacter baumannii (n = 29) from patients with VAP were exposed over time to levofloxacin, imipenem, colistin and their combinations. Synergy between levofloxacin and imipenem was found in 55.3 % and between levofloxacin and colistin in 90.9 % of isolates of P. aeruginosa within the first 4 h of growth. Synergy with imipenem but not with colistin was dependent of the MIC. Synergy between levofloxacin and imipenem was found in 58.6 % of isolates of A. baumannii after 24 h of growth. Considerable synergy was found between levofloxacin and colistin, reaching 84.8 % of isolates of A.baumannii after 6 h of growth. Synergy was independent from the MIC. These results create hopes that levofloxacin can be used as combination therapy for infections by MDR bacteria.
Similar content being viewed by others
Introduction
The emergence of multidrug-resistant (MDR) and extensively drug-resistant Gram-negative bacteria in the hospital environment all over the world and the apparent lack of newer antimicrobials create an unmet medical need. Many physicians rely on combinations of antimicrobials to combat resistant pathogens. It is expected that these combinations may help prevent further resistance development and preserve antimicrobials like tigecycline and colistin that are the last option in the therapeutic arsenal [1].
One of the most threatening infections often caused by MDR Gram-negative pathogens is ventilator-associated pneumonia (VAP). Intravenous levofloxacin has been proposed as an appropriate alternative for the treatment of patients. A recent retrospective analysis of 222 patients with VAP treated with monotherapy with either levofloxacin or imipenem–cilastatin showed similar clinical and microbiological success, which, however, did not exceed 60 % of patients [2]. Similar promising results were obtained from two non-randomised studies with 10 and 12 patients, respectively [3, 4]. Although the authors of these studies support the appropriateness of levofloxacin for the empirical management of VAP, they support that levofloxacin should not be used for isolates with minimum inhibitory concentrations (MICs) exceeding 2 μg/ml [3]. This skepticism further exists when models of pharmacodynamic simulations are studied [5].
However, many pharmacokinetic studies suggest that the penetration of levofloxacin in the lung may over-exceed 2 μg/ml, provided some dose adjustments are done [6, 7]. To this end, we studied the time–kill effect of levofloxacin on a wide panel of genetically distinct pathogens of Pseudomonas aeruginosa and of Acinetobacter baumannii from patients with VAP. Levofloxacin was studied at doses equal to those achieved in the lung parenchyma; interactions with imipenem and colistin were also studied.
Materials and methods
For the conduct of the study, isolates of P. aeruginosa and of A. baumannii isolated from the tracheobronchial secretions (TBS) of well-characterised cases of VAP were used. They were isolated at counts greater than 105 cfu/ml from the TBS of patients enrolled in two previously described studies [8, 9]. In both studies, VAP was diagnosed by the following criteria: (a) signs of sepsis; (b) new or persistent consolidation in chest X-ray; (c) purulent TBS; and (d) Clinical Pulmonary Infection Score (CPIS) more than 6. Isolates were kept frozen in skim milk at −70 °C. The studied clinical isolates derived from consented individuals who have participated in the two aforementioned clinical studies [8, 9] that were approved by the Hospital Ethics Committees and the current in vitro study was approved by the Ethics Committees of ATTIKON University Hospital.
The selection of isolates was done by two criteria from a large panel of isolates coming from the aforementioned studies [8, 9] that were kept refrigerated: (a) being genetically distinct. This was defined after pulsed-field gel electrophoresis (PFGE) of the total DNA. Briefly, after bacterial cell lysis by sonication, genomic DNA was isolated, digested by restriction endonuclease SpeI for P. aeruginosa and ApaI for A. baumannii, and subjected to electrophoresis on 1.5 % agarose gel; the voltage was adjusted to 200 V, temperature to 12 °C and both phases of ramping at 15 and 23 h in a Gene Navigator apparatus (Pharmacia Biotech, Uppsala, Sweden); and (b) being MDR. This was defined as resistance to at least three antimicrobials of different chemical classes, according to the Clinical and Laboratory Standards Institute (CLSI) criteria. Finally, 47 isolates of P. aeruginosa and 29 isolates of A. baumannii were selected for the study.
Purified powders of imipenem and of colistin sulfate were provided by Sigma-Aldrich Co. (St. Louis, MO, USA). Amorphous crystalline powder of levofloxacin was provided by Sanofi (Paris, France). The MICs of levofloxacin, imipenem and colistin were determined by a microdilution technique of a 0.1-ml final volume using a 5 × 105 cfu/ml log-phase inoculum. The MIC was considered the lowest antimicrobial concentration limiting visible bacterial growth after overnight incubation at 35 °C. Minimum bactericidal concentrations (MBCs) were determined by sub-culture of the content of clear wells onto MacConkey agar. The MBC was considered the lowest antimicrobial concentration killing at least 99 % of the plated inoculum.
In order to study the time–kill effect of levofloxacin and its interactions with imipenem and colistin, single colonies of the studied isolates were left to grow into visible turbidity in cation-adjusted Mueller–Hinton broth (CAMHB, Oxoid Ltd., London, UK) at 35 °C and then adjusted to the desired inoculum by a 0.5 McFarland standard. After dilutions, a log-phase 1 × 106 cfu/ml inoculum was exposed over time into tubes of a 10-ml final volume with levofloxacin at final concentrations of 7.5, 11 and 25 μg/ml without/with 16 μg/ml of imipenem and 5 μg/ml of colistin. Tubes were left to incubate at 37 °C in a shaking water bath and, at standard intervals (0, 2, 4, 6 and 24 h), the bacterial growth was measured in duplicate by four serial 1:10 dilutions of one 0.1-ml aliquot in 0.9 % NaCl and by plating 0.1 ml of each dilution onto MacConkey agar. The use of serial dilutions eliminated any antimicrobial carry-over effect. The lower detection limit was 10 cfu/ml. The absolute number of bacterial counts per time of growth was measured by means of the counts in each dilution; to this end, counts were multiplied with the appropriate dilution factor. Concentrations of 7.5 and 11 μg/ml of levofloxacin were selected because they are equal to those estimated in the epithelial lining fluid (ELF) 4 to 6 h after the intravenous administration of 750 and 1,000 mg of the drug, respectively [7, 10]. The concentration of 25 μg/ml of levofloxacin was studied because it is equal to the concentration achieved in the ELF 1 h after the intravenous administration of 750 or 1,000 mg of the drug [7]. Imipenem was studied at 16 μg/ml and colistin was studied at 5 μg/ml because these concentrations are close to the maximal serum and ELF levels after the administration of conventional doses [11–15].
The following effects were recorded [16]: (a) killing effect as any decrease of the starting inoculum ≥3log10 and (b) synergism between two or more antimicrobials as any ≥2log10 decrease of bacterial growth compared to the most active single agent.
Changes of bacterial growth from the starting inoculum were expressed by means ± standard error of the mean (SE). Comparisons between groups were done by analysis of variance (ANOVA) with post-hoc Bonferroni analysis. Correlations between changes of bacterial counts from the baseline and the MIC of antimicrobials were done according to Spearman’s rank of order. Receiver operator characteristics (ROC) analysis was done to identify some cut-off points of the MIC of levofloxacin, imipenem or colistin that could be used to define the probability of antimicrobial synergy with specificity more than 80 % against the studied isolates. Comparisons between sub-groups of isolates defined by the MIC cut-off points were done by Student’s t-test. Any p-value below 0.05 was considered significant.
Results
The MIC50/MIC90 of levofloxacin against the studied P. aeruginosa isolates were 16/64 μg/ml, of imipenem 8/256 μg/ml and of colistin 2/32 μg/ml. The MBC50/MBC90 were 32/128 μg/ml, 16/512 μg/ml and 4/32 μg/ml, respectively.
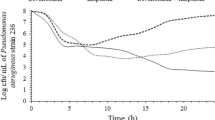
Levofloxacin achieved a significant decrease of bacterial growth of P. aeruginosa; this was pronounced when the tested concentration was 25 μg/ml (Fig. 1a). A time–kill effect of levofloxacin was shown against 53.2 % of the studied isolates within the first 4 h of incubation (Table 1). At this time point, negative correlations were found between the MIC of levofloxacin and the decrease of bacterial counts of P. aeruginosa. These correlations were shown when the studied concentration was 7.5 μg/ml (r s: −0.618, p < 0.0001), when the studied concentration was 11 μg/ml (r s: −0.519, p < 0.0001) and when the studied concentration was 25 μg/ml (r s: −0.571, p < 0.0001).
Change of bacterial counts of (a) 47 isolates of Pseudomonas aeruginosa and (b) 29 isolates of Acinetobacter baumannii over time after in vitro exposure to 7.5 μg/ml of levofloxacin (LVF7.5), 11 μg/ml of levofloxacin (LVF11), 25 μg/ml of levofloxacin (LVF25), 16 μg/ml of imipenem (IMP) and 5 μg/ml of colistin (COL). Thirty-three of the studied isolates of P. aeruginosa and 13 of the studied isolates of A. baumannii were tested on colistin. p-Value of comparisons at the indicated time intervals after correction for multiple comparisons by Bonferroni for significant differences: a0.001 LVF25 vs. IMP, b0.008 LVF11 vs. IMP, c0.044 LVF25 vs. COL, d0.004 COL vs. all LVF concentrations, e < 0.0001 LVF25 vs. LVF11/LVF7.5
The time interactions of levofloxacin with imipenem were studied against all 47 isolates of P. aeruginosa and of levofloxacin with colistin against 33 isolates of P. aeruginosa. Synergistic effects between levofloxacin and imipenem were found in 55.3 % of the studied isolates and between levofloxacin and colistin in 90.9 % of isolates within the first 4 h of incubation (Table 2). ROC analysis showed that an MIC of levofloxacin of ≤32 μg/ml and an MIC of imipenem ≤16 μg/ml was linked with specificity more than 80 % to predict synergy at 4 h between levofloxacin and imipenem against P. aeruginosa. Further analysis revealed that the change of bacterial growth by the antimicrobial interaction was independent of the MIC of levofloxacin when the MIC of imipenem was ≤16 μg/ml; however, the decrease of bacterial growth for isolates with an MIC of imipenem >16 μg/ml was greater when the MIC of levofloxacin was ≤32 μg/ml compared to >32 μg/ml (Fig. 2). ROC analysis failed to define some similar MIC cut-off points of colistin predictive of the synergy between levofloxacin and colistin against P. aeruginosa (data not shown). Representative time–kill curves for four isolates are shown in Fig. 3.
Correlations between the minimum inhibitory concentration (MIC) of levofloxacin and imipenem, and the change of P. aeruginosa growth after 4 h of incubation. Isolates are divided according to MICs of imipenem (IMP) of ≤16 μg/ml and >16 μg/ml, and according to MICs of levofloxacin (LVF) of ≤32 μg/ml and >32 μg/ml. Changes of bacterial growth after 4 h of growth are shown (a) in the presence of 7.5 μg/ml of LVF and IMP, (b) in the presence of 11 μg/ml of LVF and IMP, and (c) in the presence of 25 μg/ml of LVF and IMP. p-Values refer to statistical comparisons between isolates with MICs of LVF of ≤32 μg/ml and >32 μg/ml
The MIC50/MIC90 of levofloxacin against the studied isolates of A. baumannii were 16/32 μg/ml, of imipenem 16/64 μg/ml and of colistin 0.5/2 μg/ml. The MBC50/MBC90 were 16/64 μg/ml, 32/64 μg/ml and 1/2 μg/ml, respectively. No significant time–kill effect of levofloxacin on A. baumannii was found (Fig. 1b and Table 1). The time–kill effect was limited in 27.6 % of the studied isolates.
The time interactions of levofloxacin with imipenem were studied against all 29 isolates of A. baumannii and of levofloxacin with colistin against 13 isolates of A. baumannii. Synergy between levofloxacin and imipenem was found in 58.6 % of the studied isolates after 24 h of incubation (Table 2). Considerable synergy was found between levofloxacin and colistin, reaching 84.8 % of the studied isolates after 6 h of incubation. ROC analysis failed to define some MIC cut-off points of levofloxacin, of imipenem or of colistin predictive of the synergy between levofloxacin and imipenem or between levofloxacin and colistin against A. baumannii (data not shown). Representative time–kill curves for three isolates of A. baumannii are shown in Fig. 4.
Discussion
The presented results provide evidence that levofloxacin, when administered at doses that can deliver lung concentrations between 11 and 25 μg/ml, may possess a considerable time–kill effect on MDR P. aeruginosa pathogens derived from patients with VAP. Levofloxacin exhibits considerable synergy with imipenem and colistin against that species. The efficacy of the in vitro interaction with imipenem is greater as the MIC levels of levofloxacin and imipenem decrease. On the contrary, the time–kill effect of levofloxacin on A. baumannii is limited.
Although originally described as an extremely potent agent against P. aeruginosa, antimicrobial susceptibility testing of isolates from blood, urine and respiratory specimens from the intensive care units of 19 medical centres of Canada during 2005 and 2006 showed that levofloxacin was in vitro active against 68 % of P. aeruginosa isolates [17]. A total of 419 isolates was studied; the MIC50 of levofloxacin was 1 μg/ml and that of meropenem was 1 μg/ml. Using these results, it can be postulated that our findings are important in the clinical setting for two reasons: (i) the MIC levels of the 47 studied isolates are greater than those reported by the Canadian study and (ii) the synergistic effect between levofloxacin and imipenem was shown as early as 4 h after exposure of the bacterial inoculum to this interaction.
A more recent study has assessed the in vitro interaction of β-lactams with levofloxacin against P. aeruginosa but comprised only four isolates. In this study, isolates with MICs of levofloxacin between 1 and 4 μg/ml were exposed to concentrations equal to 0.5×, 1× and 4× the MIC in combination with ceftobiprole. Synergy was shown against three of the isolates starting as early as 6 h after antimicrobial exposure [18].
To test the pharmacodynamics of antimicrobials, the hollow-fibre infection model has been developed. In that model, antimicrobials are infused in the central compartment and bacterial killing or emergence of resistance is monitored in the peripheral compartments. Using this model, the efficacy of levofloxacin in combination with meropenem was tested against a wild-type P. aeruginosa PAO1 strain and against a strain over-expressing the MexAB pump. The results showed that single antimicrobials did not manage to eradicate the strains and prevent the emergence of resistance. However, the combination achieved rapid bacterial killing from the first day and prevented the emergence of resistance [19]. In order to act in synergy, the authors found that two conditions should apply: (a) the ratio of the minimum concentration to the MIC of meropenem should be close to 1 and (b) the ratio of the area under the curve to the MIC of levofloxacin should be close to 31. These conditions apply when the regimen tested was at least 750 mg once daily for levofloxacin and at least 3 g three times daily for meropenem. These regimens deliver ranges of concentrations within those studied in our study [7, 10], making our results extremely promising in the clinical field.
The pharmacodynamics of levofloxacin in a murine model of pneumonia induced by P. aeruginosa PAO1 creates skepticism as to whether levofloxacin can be used as monotherapy for VAP. The lung penetration of levofloxacin was 77 % and the decrease of viable counts was greater as the administered dose increased. Data from this model were used to predict the ability of levofloxacin to eradicate P. aeruginosa from the lung in humans by a single daily dose of 750 mg based on Monte Carlo simulation. The results suggested that this was less possible for strains with MIC values greater than 1 μg/ml [19].
A large-scale randomised clinical trial was conducted to compare the clinical efficacy of monotherapy with levofloxacin over imipenem for hospital-acquired pneumonia. Sub-group analysis was done regarding patients with VAP. The clinical success rate in the levofloxacin arm was 58.6 % [2]. The limited clinical efficacy and the pharmacodynamic results reported previously [19, 20] underscore the need to use levofloxacin only in combination. A small-scale, single-arm, open-label study was published where nine patients with VAP caused by P. aeruginosa were treated with the combination of levofloxacin 500 mg twice daily and ceftazidime 2 g twice daily; VAP was eradicated in eight of these cases [21].
Although levofloxacin is considered in vitro active against A. baumannii [17], the available data in the literature are limited. One study has described synergy between cefepime and levofloxacin against an MDR isolate using the hollow-fibre infection model. Synergism was pronounced as drug concentrations increased [22]. In our study, synergy between levofloxacin and imipenem was found against 58.6 % of MDR A. baumannii pathogens. However, this was shown only after 24 h of incubation and when the studied concentration of levofloxacin was 25 μg/ml. These findings do not encourage the use of this combination for infections by this species.
Another interesting finding of our study was the in vitro synergy of levofloxacin and colistin against both P. aeruginosa and A. baumannii, which is described for the first time in the literature. Colistin in both parenteral and aerolised administration is, nowadays, the treatment of choice for infections by these species. Most people believe that colistin should not be given as monotherapy, with the aim to avoid the emergence of resistance [23]. The observed synergy with levofloxacin provides a very good choice for clinicians who wish to select a second antimicrobial for co-administration, particularly since synergy does not depend on the MIC of colistin or levofloxacin.
The presented results create hopes that levofloxacin can be used as combination therapy for serious infections by MDR species of P. aeruginosa and A. baumannii. In the case of P. aeruginosa, levofloxacin synergises dynamically with imipenem and the effect largely depends on the MIC level. The interaction of levofloxacin and imipenem is poorly active on A. baumannii. However, powerful synergy between levofloxacin and colistin is found against both species. The observed synergy effects of levofloxacin with colistin and imipenem and their clinical benefits should be confirmed in future clinical trials.
References
Fischbach MA (2011) Combination therapies for combating antimicrobial resistance. Curr Opin Microbiol 14:519–523
Shorr AF, Zadeikis N, Jackson WL, Ramage AS, Wu SC, Tennenberg AM, Kollef MH (2005) Levofloxacin for treatment of ventilator-associated pneumonia: a subgroup analysis from a randomized trial. Clin Infect Dis 40(Suppl 2):S123–S129
Benko R, Matuz M, Doro P, Peto Z, Molnar A, Hajdu E, Nagy E, Gardi J, Soos G (2007) Pharmacokinetics and pharmacodynamics of levofloxacin in critically ill patients with ventilator-associated pneumonia. Int J Antimicrob Agents 30:162–168
Pea F, Di Qual E, Cusenza A, Brollo L, Baldassarre M, Furlanut M (2003) Pharmacokinetics and pharmacodynamics of intravenous levofloxacin in patients with early-onset ventilator-associated pneumonia. Clin Pharmacokinet 42:589–598
Hutschala D, Skhirtladze K, Zuckermann A, Wisser W, Jaksch P, Mayer-Helm BX, Burgmann H, Wolner E, Müller M, Tschernko EM (2005) In vivo measurement of levofloxacin penetration into lung tissue after cardiac surgery. Antimicrob Agents Chemother 49:5107–5111
Conte JE Jr, Golden JA, McIver M, Little E, Zurlinden E (2007) Intrapulmonary pharmacodynamics of high-dose levofloxacin in subjects with chronic bronchitis or chronic obstructive pulmonary disease. Int J Antimicrob Agents 30:422–427
Conte JE Jr, Golden JA, McIver M, Zurlinden E (2006) Intrapulmonary pharmacokinetics and pharmacodynamics of high-dose levofloxacin in healthy volunteer subjects. Int J Antimicrob Agents 28:114–121
Giamarellos-Bourboulis EJ, Pechère JC, Routsi C, Plachouras D, Kollias S, Raftogiannis M, Zervakis D, Baziaka F, Koronaios A, Antonopoulou A, Markaki V, Koutoukas P, Papadomichelakis E, Tsaganos T, Armaganidis A, Koussoulas V, Kotanidou A, Roussos C, Giamarellou H (2008) Effect of clarithromycin in patients with sepsis and ventilator-associated pneumonia. Clin Infect Dis 46:1157–1164
Gogos C, Kotsaki A, Pelekanou A, Giannikopoulos G, Vaki I, Maravitsa P, Adamis S, Alexiou Z, Andrianopoulos G, Antonopoulou A, Athanassia S, Baziaka F, Charalambous A, Christodoulou S, Dimopoulou I, Floros I, Giannitsioti E, Gkanas P, Ioakeimidou A, Kanellakopoulou K, Karabela N, Karagianni V, Katsarolis I, Kontopithari G, Kopterides P, Koutelidakis I, Koutoukas P, Kranidioti H, Lignos M, Louis K, Lymberopoulou K, Mainas E, Marioli A, Massouras C, Mavrou I, Mpalla M, Michalia M, Mylona H, Mytas V, Papanikolaou I, Papanikolaou K, Patrani M, Perdios I, Plachouras D, Pistiki A, Protopapas K, Rigaki K, Sakka V, Sartzi M, Skouras V, Souli M, Spyridaki A, Strouvalis I, Tsaganos T, Zografos G, Mandragos K, Klouva-Molyvdas P, Maggina N, Giamarellou H, Armaganidis A, Giamarellos-Bourboulis EJ (2010) Early alterations of the innate and adaptive immune statuses in sepsis according to the type of underlying infection. Crit Care 14:R96
Rodvold KA, Danziger LH, Gotfried MH (2003) Steady-state plasma and bronchopulmonary concentrations of intravenous levofloxacin and azithromycin in healthy adults. Antimicrob Agents Chemother 47:2450–2457
Adamis G, Papaioannou MG, Giamarellos-Bourboulis EJ, Gargalianos P, Kosmidis J, Giamarellou H (2004) Pharmacokinetic interactions of ceftazidime, imipenem and aztreonam with amikacin in healthy volunteers. Int J Antimicrob Agents 23:144–149
Li C, Nicolau DP, Lister PD, Quintiliani R, Nightingale CH (2004) Pharmacodynamic study of beta-lactams alone and in combination with beta-lactamase inhibitors against Pseudomonas aeruginosa possessing an inducible beta-lactamase. J Antimicrob Chemother 53:297–304
Drusano GL, Lodise TP, Melnick D, Liu W, Oliver A, Mena A, VanScoy B, Louie A (2011) Meropenem penetration into epithelial lining fluid in mice and humans and delineation of exposure targets. Antimicrob Agents Chemother 55:3406–3412
Li J, Rayner CR, Nation RL, Deans R, Boots R, Widdecombe N, Douglas A, Lipman J (2005) Pharmacokinetics of colistin methanesulfonate and colistin in a critically ill patient receiving continuous venovenous hemodiafiltration. Antimicrob Agents Chemother 49:4814–4815
Athanassa ZE, Markantonis SL, Fousteri MZF, Myrianthefs PM, Boutzouka EG, Tsakris A, Baltopoulos GJ (2012) Pharmacokinetics of inhaled colistimethate sodium (CMS) in mechanically ventilated critically ill patients. Intensive Care Med 38:1779–1786
Giamarellos-Bourboulis EJ, Kentepozidis N, Antonopoulou A, Plachouras D, Tsaganos T, Giamarellou H (2005) Postantibiotic effect of antimicrobial combinations on multidrug-resistant Pseudomonas aeruginosa. Diagn Microbiol Infect Dis 51:113–117
Zhanel GG, DeCorby M, Nichol KA, Wierzbowski A, Baudry PJ, Karlowsky JA, Lagacé-Wiens P, Walkty A, Mulvey MR, Hoban DJ; Canadian Antimicrobial Resistance Alliance (2008) Antimicrobial susceptibility of 3931 organisms isolated from intensive care units in Canada: Canadian National Intensive Care Unit Study, 2005/2006. Diagn Microbiol Infect Dis 62:67–80
Kresken M, Körber-Irrgang B, Läuffer J, Decker-Burgard S, Davies T (2011) In vitro activities of ceftobiprole combined with amikacin or levofloxacin against Pseudomonas aeruginosa: evidence of a synergistic effect using time–kill assay methodology. Int J Antimicrob Agents 38:70–75
Louie A, Grasso C, Bahniuk N, Van Scoy B, Brown DL, Kulawy R, Drusano GL (2010) The combination of meropenem and levofloxacin is synergistic with respect to both Pseudomonas aeruginosa kill rate and resistance suppression. Antimicrob Agents Chemother 54:2646–2654
Louie A, Fregeau C, Liu W, Kulawy R, Drusano GL (2009) Pharmacodynamics of levofloxacin in a murine pneumonia model of Pseudomonas aeruginosa infection: determination of epithelial lining fluid targets. Antimicrob Agents Chemother 53:3325–3330
Bassetti M, Righi E, Rosso R, Mannelli S, Di Biagio A, Fasce R, Pallavicini FB, Marchetti F, Viscoli C (2006) Efficacy of the combination of levofloxacin plus ceftazidime in the treatment of hospital-acquired pneumonia in the intensive care unit. Int J Antimicrob Agents 28:582–585
Lim TP, Ledesma KR, Chang KT, Hou JG, Kwa AL, Nikolaou M, Quinn JP, Prince RA, Tam VH (2008) Quantitative assessment of combination antimicrobial therapy against multidrug-resistant Acinetobacter baumannii. Antimicrob Agents Chemother 52:2898–2904
Biswas S, Brunel JM, Dubus JC, Reynaud-Gaubert M, Rolain JM (2012) Colistin: an update on the antibiotic of the 21st century. Expert Rev Anti Infect Ther 10:917–934
Source of funding
The study was supported by an unrestricted educational grant by Sanofi S.A., Athens, Greece.
Conflict of interest
None of the authors have a conflict of interest related with this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Safarika, A., Galani, I., Pistiki, A. et al. Time–kill effect of levofloxacin on multidrug-resistant Pseudomonas aeruginosa and Acinetobacter baumannii: synergism with imipenem and colistin. Eur J Clin Microbiol Infect Dis 34, 317–323 (2015). https://doi.org/10.1007/s10096-014-2231-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-014-2231-7