Abstract
We investigated the decomposition of cellulose upon heating in an ionic liquid, 1-ethyl-3-methylimidazolium chloride ([C2mim][Cl]), in air and we studied the reactions of various cellulose decomposition compounds such as cellobiose, cellobiosan, glucose and levoglucosan. We propose two possible decomposition pathways for cellulose during [C2mim][Cl] treatment in air. One pathway is the hydrolysis of cellulose to cellobiose and glucose because [C2mim][Cl] can retain water even at higher than 100 °C, the boiling point of water. The other pathway is the depolymerization of cellulose to cellobiosan by a reaction of the hydroxy group at the C6 position in the glucose residue to form a glycosidic bond. The obtained cellobiosan is then depolymerized to two molecules of levoglucosan by a similar reaction of the hydroxy group at the C6 position. The levoglucosan produced is further hydrolyzed to glucose. Therefore, several products from cellulose decomposition are converted to glucose, which is the final hydrolyzed product. Furthermore, the obtained glucose can be further dehydrated to 5-HMF.
Similar content being viewed by others
Introduction
Recently, several energy and environmental problems from the use of fossil resources have been identified. To solve these problems and establish a sustainable society based on renewable resources, the efficient use of biomass is required.
Among the various types of biomass, lignocellulosics such as wood account for more than 90 % of the biomass in the world. Lignocellulosics are renewable and do not compete with food resources. To produce various useful materials from lignocellulosics, methods such as acid hydrolysis [1], enzymatic saccharification [2], pyrolysis [3, 4] and supercritical fluid treatment [5] have been studied. These methods, however, require severe conditions for treatment such as high temperatures and high pressures.
Recently, much attention has been given to treatment using ionic liquids as an attractive novel technology for the conversion of lignocellulosics. Ionic liquids are organic salts with low melting points around ambient temperatures [6] and they are electrochemically stable [7]. They are only composed of cations and anions. By the modification of these cations and/or anions, a wide range of potential ionic liquids can be tailored to display a wide variety of solvent properties. Furthermore, ionic liquids are regarded as “green solvents” because of their negligible vapor pressure, non-flammability and recyclability.
Cellulose is the main component of wood and its solubility in various solvent systems has been investigated [8–11]. Recently, it has been revealed that some ionic liquids can dissolve cellulose [12]. They are expected as green solvent because of their many unique characteristics, such as negligible vapor pressure, thermal stability, reusability, and non-flammability.
Many fundamental and applied studies have focused on various ionic liquid treatments of cellulose for chemical conversion since Swatloski et al. [12] revealed that imidazolium-based ionic liquids can dissolve cellulose. Ionic liquids comprising imidazolium cations and chloride or acetate anions are often used in cellulose research. Vitz et al. [13] reported that cellulose dissolves in 1-butyl-3-methylimidazolium chloride ([C4mim][Cl]) and 1-ethyl-3-methylimidazolium chloride ([C2mim][Cl]), but that [C4mim][Cl] gives a higher degree of cellulose degradation than [C2mim][Cl]. The influence of water on the dissolution of cellulose in 1-ethyl-3-methylimidazolium acetate ([C2mim][Ac]) was analyzed by Le et al. [14]. They reported that cellulose does not completely dissolve in [C2mim][Ac], if it contains more than 15 wt% water. The dissolution of cellulose is greatly affected by the source of cellulose [15], the water content [16], the series of ionic liquids used [17–19] and other conditions such as the heating method and the temperature. The dissolution mechanism of cellulose in ionic liquids has been partly determined. NMR analysis suggests that the solubilization of cellulose in ionic liquids is mainly caused by hydrogen bonding between the hydroxyl groups of cellulose and the chloride or acetate anions and very little interaction exists with the imidazolium cations [20–22]. These interactions were also investigated by molecular dynamics simulations [23, 24].
Cellulose solubilized in [C4mim][Cl] or [C2mim][Ac] can be recovered as amorphous cellulose by the addition of anti-solvents for cellulose (such as water) [25, 26]. These regenerated celluloses show enhanced activity during enzymatic hydrolysis by cellulase, compared with native cellulose [26, 27]. Regenerated cellulose can be obtained as films and these films contain crystals of cellulose II, which are different from native cellulose [28, 29]. Nitrogen is not detected in dried films obtained from 1-allyl-3-methylimidazolium chloride [30] and [C2mim][Cl] [31] upon the addition of distilled water.
Ionic liquids have also been used as alternative solvents for the derivatization of cellulose such as acetylation [32, 33], carboxymethylation [34], tritylation [17] and succination [35], since conventional organic solvents have limitations because of volatility, toxicity and difficulties of recovery. In addition, much work has been done to determine the most efficient reaction conditions for cellulosic conversion to 5-hydroxymethylfurfural [36–39] and sorbitol [40] in ionic liquids.
A higher cellulose depolymerization rate during ionic liquid dissolution occurs in [C4mim][Cl] and [C2mim][Cl] compared with [C2mim][Ac] [15]. [C2mim][Cl] can dissolve and decompose cellulose into cellobiose, cellobiosan, glucose, levoglucosan and 5-hydroxymethylfurfural. In addition, [C2mim][Cl] can also react with the compounds produced by cellulose to form new polymers [31]. However, the detailed reaction decomposition behavior of cellulose in ionic liquids remains unclear.
In this study, therefore, the decomposition behavior of cellulose in [C2mim][Cl] was examined in detail by studying the reaction behavior of low molecular compounds such as cellobiose, cellobiosan, glucose and levoglucosan in [C2mim][Cl]. This fundamental information is crucial in understanding the reaction behavior of wood polysaccharides and applying ionic liquids to the effective use of wood.
Materials and methods
Samples and chemicals
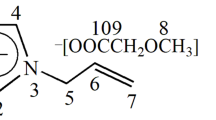
Cellobiose, glucose, levoglucosan and 5-hydroxymethylfurfural (5-HMF) were purchased from Wako Pure Chemical Industries, Ltd., Osaka, Japan. Cellobiosan was purchased from Carbosynth Ltd., Berkshire, UK. All compounds were dried in an oven at 105 °C for 24 h before use. Water-18O as stable isotopes (SI) (purity 99.99 %) was purchased from Taiyo Nippon Sanso, Tokyo, Japan. The ionic liquid, 1-ethyl-3-methylimidazolium chloride ([C2mim][Cl]), was purchased from Tokyo Chemical Industry Co., Ltd., Tokyo, Japan.
Treatment with [C2mim][Cl]
The 3 g of [C2mim][Cl] was heated at 100 °C in a 100-ml glass flask. The reaction atmosphere in the flask was controlled by vacuum, nitrogen (N2) flow or air flow. After melting the [C2mim][Cl], 0.09 g of cellobiose, cellobiosan, glucose and levoglucosan were added to the flask and the reaction media were gently stirred. The point where the reaction media became transparent without any particles in the flask was defined by 0 h of treatment. It took several minutes before the reaction media became transparent at 100 °C.
Evaluation methods
Fourier transform infrared (FT-IR) spectra of [C2mim][Cl] were recorded using an IRAffinity-1 spectrophotometer (Shimadzu, Kyoto, Japan), over a spectral range of 4000–400 cm−1 with 32 accumulations. A GladiATR Vision Diamond Crystal Plate (ST Japan INC.) was used as a stage heater. Dry nitrogen (N2) gas was directed to flow to the sample stage. In case of making N2 gas humidify, the humidity was contained in N2 gas flow by having it pass through distilled water in midflow. The temperature program was 20 °C/min from 37.5 to 120 °C.
To determine the influence of water on the reaction of cellulose in [C2mim][Cl], a Shimadzu GCMS-QP2010 ultra gas chromatography–mass spectrometer (GC–MS) was used. The samples for GC–MS analysis were prepared as follows: 20 µl of the reaction media were homogeneously mixed with 240 µl of acetonitrile. After drying with sodium sulfate, the obtained mixture was filtered using a 0.45-µm filter. The 120 µl of the filtrate was silylated at room temperature using 60 µl of BSTFA mixed with 10 µl pyridine. The obtained silylated samples were analyzed by GC–MS. The capillary column was a ULBON HR-52 (diameter 0.25 mm) from Shinwa Chemical Industries Ltd., Kyoto, Japan. The temperature program was 60 °C (0 → 1 min), 60 → 230 °C (1 → 21 min) and 230 °C (21 → 50 min). Helium was used as a carrier gas at a flow rate of 1.0 ml/min. The injector and detector temperatures were 250 and 230 °C, respectively.
The compounds solubilized in [C2mim][Cl] were analyzed by high performance liquid chromatography (HPLC). HPLC was carried out on a Shimadzu Prominence, equipped with pump (LC-20AD), column oven (CTO-20A) and refractive index director (RID-10A). The samples for this analysis were prepared as follows: at a specific reaction time, 20 µl reaction medium was homogeneously mixed with 180 µl distilled water and then filtered through a 0.45-µm filter. The filtrates were analyzed as follows: column, Shodex Sugar KS-801 (SHOWA DENKO, Tokyo, Japan); flow rate, 1 ml/min; eluent, distilled water; detector, refractive index director (RID); column temperature, 80 °C.
Results and discussion
Water in [C2mim][Cl]
In our previous work [31], we found that the hydrolysis of cellulose occurred in [C2mim][Cl] at 100–140 °C, which is higher than the boiling point of water, and we identified various hydrolyzed products of cellulose such as cellooligosaccharides, cellobiose and glucose. Thus, the presence of water in [C2mim][Cl] was studied under various conditions.
Figure 1 shows the infrared spectra for [C2mim][Cl] under various conditions. The atmospheric and temperature conditions were changed as shown in Fig. 1a. The temperature program was 37.5 → 120 °C (0 → 5 min) and 120 °C (5 → 35 min). The atmosphere consisting of humidified nitrogen (0 → 15 min) is indicated as “N2 + H2O”, and dry nitrogen (15 → 25 min) is indicated as “N2” followed by humidified nitrogen (25 → 35 min). The IR spectra were recorded under various conditions as indicated by (1), (2), (3) and (4). The spectra measured under N2 + H2O at 37.5 °C (condition (1)) show a water peak at 3400 cm−1, as shown in Fig. 1b. The obvious peak at 3400 cm−1 is present despite heating at 120 °C under N2 + H2O (condition (2)). This result reveals that the water in [C2mim][Cl] under N2 + H2O remains even at 120 °C, which is higher than the boiling point of water. The spectrum obtained for condition (3) does not show a peak at 3400 cm−1. Therefore, under dry N2 [C2mim][Cl] the water in [C2mim][Cl] can be evaporated. Although noise peaks over 3400 cm−1 can be seen, it is not clear why these peaks appeared. For N2 + H2O (condition (4)), the peak at 3400 cm−1 is present. These results indicate that [C2mim][Cl] can absorb moisture from the reaction atmosphere and retain it, even at 120 °C, which is higher than the boiling point of water.
Influence of water on hydrolysis in [C2mim][Cl]
To study the influence of the water absorbed by [C2mim][Cl] from the atmosphere on hydrolysis by [C2mim][Cl], cellobiose was treated in [C2mim][Cl] at 100 or 120 °C with the experimental apparatus shown in Fig. 2. N2 was directed into a flask containing water-18O, and then humidified N2 gas (N2 + water-18O) was flowed into the reaction flask that contained [C2mim][Cl] and cellobiose to fill the reaction atmosphere with N2 + water-18O. After treatment at 100 or 120 °C, the glucose produced from cellobiose was analyzed by HPLC and GC–MS. Under this reaction, cellobiose is hydrolyzed to produce two molecules of glucose. Water is used for cleavage of glycosidic bond in cellobiose, and 18O can be included in either glucose produced.
Figure 3 shows the mass spectrum of the trimethylsilyl derivative of the glucose produced from the cellobiose treated in [C2mim][Cl] at 100 °C for 48 h (a) or at 120 °C for 24 h (b) under N2 + water-18O flow. This was compared with that produced under humidified N2 gas flow with normal water (N2 + water-16O). In spectrum (c), the characteristic ions are present at m/z 271, 291, 345, 361, 393 and 454. These are typical ions for trimethylsilylated glucose. In spectra (a) and (b), however, not only those ions but also ions at 273, 293, 347, 363, 395 and 456 were detected. The difference in m/z between the former and latter is two, which corresponds to the difference in the molecular weight for water-18O and water-16O. Thus, water-18O in the reaction atmosphere was used for the hydrolysis of cellobiose in [C2mim][Cl]. From the results in Figs. 1 and 3, [C2mim][Cl] absorbs moisture even at higher than 100 °C, and this absorbed water can induce hydrolysis in [C2mim][Cl].
Reaction pathway for the production of cellobiosan from cellulose by [C2mim][Cl] treatment
In our previous paper, cellulose was found to decompose into cellobiosan during [C2mim][Cl] treatment [31]. Two reaction pathways are proposed to explain the formation of cellobiosan from cellulose, as shown in Fig. 4. Pathway 1 shows the dehydration of cellobiose, which is produced by the hydrolysis of cellulose. Conversely, pathway 2 shows that cellulose is directly decomposed to cellobiosan. The hydroxy group at the C6 position of the glucose residue interacts with the glycosidic bond and the glycosidic linkages cleave to form cellobiosan.
To confirm pathway 1, as shown in Fig. 4, the reaction behavior of cellobiose during [C2mim][Cl] treatment was studied. HPLC analyses were performed for the compounds obtained from cellobiose after the treatment of cellobiose at 100 °C under air. Figure 5 shows changes in the yields of the various compounds produced. The yield of cellobiose decreases as the treatment time is extended. In contrast, the yields of glucose, levoglucosan and 5-hydroxymethylfurfural (5-HMF) increase with prolonged treatment time.
The yield of cellobiosan was negligible even after 48 h, indicating that cellobiosan cannot be produced through the dehydration of cellobiose as shown in pathway 1, but may be produced through pathway 2. To study the detailed decomposition of cellobiose, the treatment of cellobiose with [C2mim][Cl] was performed under vacuum or N2 gas flow to create a reaction atmosphere with less humidity.
Table 1 shows the yields of various products from cellobiose. Under vacuum, cellobiose decomposes partially to glucose without dehydrating to cellobiosan despite the lower humidity in the reaction atmosphere. The decomposition of cellobiose under N2 on the other hand is much faster than that under vacuum. Glucose as well as 5-HMF was quantified as decomposition products of cellobiose. 5-HMF is a dehydration product of glucose indicating that water can be produced in [C2mim][Cl]. Because of the higher yield of glucose under N2 compared with that under vacuum, the water produced is thought to remain in [C2mim][Cl] as mentioned above and can be used for the hydrolysis of cellobiose to glucose although it can be immediately removed from [C2mim][Cl] under vacuum. It is confirmed that 5-HMF is unstable and disappears during heating in [C2mim][Cl] (data are not shown). This may be why the yield of 5-HMF is much lower than that of glucose.
Reaction behavior of cellulose during [C2mim][Cl] treatment
Table 2 shows the yields of various products from the cellulose treated with [C2mim][Cl] at 100 °C for 72 h under various reaction atmospheres. Cellobiose, cellobiosan, glucose, levoglucosan and 5-HMF are present in [C2mim][Cl]. Cellobiosan was confirmed to be present under all reaction atmospheres, indicating that the reaction proposed as pathway 2 in Fig. 4 occurs under all reaction atmospheres. Under N2, cellobiose and glucose, which are the hydrolyzed products of cellulose, are produced in higher quantities compared with the reaction under vacuum. As described above, water can be produced during the decomposition of cellobiose. Thus, in the case of cellulose, the water produced during the decomposition of cellulose promotes the hydrolysis of cellulose in [C2mim][Cl]. Under air, water from cellulose and moisture from the air dissolve in [C2mim][Cl], as shown in Fig. 1. The water in [C2mim][Cl] under air is thought to accelerate the hydrolysis of cellulose. Therefore, a higher yield of various products was obtained under air, as listed in Table 2.
Reaction behavior of cellobiosan during [C2mim][Cl] treatment
Table 3 shows the yields of various products from cellobiosan treated with [C2mim][Cl] at 100 °C for 48 h under various reaction atmospheres. Under air, where a significant amount of water is present in [C2mim][Cl], cellobiosan decomposes to various products. The yield of glucose is equivalent to that of levoglucosan. This suggests that cellobiosan is hydrolyzed with water in [C2mim][Cl] to form glucose and levoglucosan. Under N2, where less water is present than under air, the yield of levoglucosan is higher than that of glucose under vacuum. Under vacuum, where only a small amount of water is present, the decomposed products are mainly composed of levoglucosan. The reaction where the glycosidic bond in cellobiosan is cleaved is shown in Fig. 4 and this is thought to occur to form two molecules of levoglucosan from cellobiosan. Because some cellobiose can be obtained from cellobiosan under air or N2, the hydrolysis of the levoglucosan residue in cellobiosan also occurs as a minor reaction.
Reaction behavior of glucose and levoglucosan during [C2mim][Cl] treatment
Table 4 shows the yields of various products from glucose treated with [C2mim][Cl] at 100 °C for 48 h under various reaction atmospheres. Under air and N2, the main product from glucose is 5-HMF, but 5-HMF was not detected under vacuum. This decomposition of glucose to 5-HMF was accelerated under air because the yield of glucose is less than that under vacuum or N2. The dehydration of glucose also occurs to form levoglucosan. The yield of levoglucosan and 5-HMF under air is higher than those under vacuum or N2. This is due that much glucose decomposed under air compared with under vacuum or N2. However, the yields of all products under air are low. As described above, 5-HMF is not stable in [C2mim][Cl]. Thus, produced 5-HMF is thought to be decomposed further during heating in [C2mim][Cl] although the products from 5-HMF are still unknown. Table 5 shows the yields of various products from the levoglucosan treated with [C2mim][Cl] at 100 °C for 48 h under various reaction atmospheres. The main product from levoglucosan is glucose, indicating that the hydrolysis of levoglucosan occurred. However, some levoglucosan remains even after 48 h of treatment under all reaction atmospheres. These results indicate that levoglucosan is more stable in [C2mim][Cl] than glucose. Under air and N2, 1.6 and 0.1 % 5-HMF were produced from glucose, respectively, because the dehydration of glucose occurred during treatment with [C2mim][Cl].
Decomposition pathway of cellulose in [C2mim][Cl]
Based on the results obtained in this study, two possible decomposition pathways for cellulose during [C2mim][Cl] treatment under air are shown in Fig. 6. The pathways A and B in this figure show not only the decomposition to cellobiosan as in pathways 1 and 2 in Fig. 4 but also whole decomposition pathway of cellulose including the decomposition to cellobiosan. In pathway A, cellulose is depolymerized to cellooligosaccharides, cellobiose and glucose upon hydrolysis with the moisture absorbed by [C2mim][Cl] from the air. The obtained glucose is converted to 5-HMF with the production of water. This water further reacts and contributes to the depolymerization of cellulose, cellooligosaccharides and cellobiose. In pathway B, cellulose is depolymerized to cellobiosan by the reaction of the hydroxy group at the C6 position in the glucose residue to give a glycosidic bond. The obtained cellobiosan is depolymerized into two molecules of levoglucosan by a similar reaction of the hydroxy group at the C6 position. The levoglucosan produced is further hydrolyzed to glucose. As for the other reaction of cellobiosan, it is hydrolyzed to glucose and levoglucosan, which is further hydrolyzed to glucose as mentioned above. The produced glucose is converted to 5-HMF, as shown in pathway A. However, the reaction of the hydroxyl group at the C6 position shown in pathway B is expected to occur randomly in cellulose. Therefore, cellobiosan in addition to several 1,6-anhydro-β-d-cellooligosaccharides such as cellotriosan, cellotetraosan and cellopentaosan must be produced. Considering these aspects and all results shown above, a decomposition pathway of cellulose in [C2mim][Cl] is proposed as shown in Fig. 7.
Conclusions
The decomposition of cellulose by heating in [C2mim][Cl] under various atmospheres was studied, and a decomposition pathway is proposed. Although a pathway for the production of anhydrosugars exists, hydrolysis is superior because [C2mim][Cl] can retain water, even at higher than 100 °C, the boiling point of water. Several products from cellulose were found to be converted to glucose, which was the final hydrolyzed product. Furthermore, the obtained glucose was further dehydrated to 5-HMF. Consequently, hydrolysis coexists with dehydration during the decomposition of cellulose in [C2mim][Cl] under air. The conclusion in this study implies that water content in the reaction system of [C2mim][Cl] is important to control the reaction of cellulose in [C2mim][Cl]. Under the reaction condition with much water content, the hydrolysis of cellulose can be accelerated to obtain several useful low molecular weight compounds. Under less water content, on the other hand, cellulose can be dissolved in [C2mim][Cl] without decomposition.
References
Goldstein IS (1980) The hydrolysis of wood. TAPPI 63:141–143
Chang VS, Holtzapple MT (2000) Fundamental factors affecting biomass enzymatic reactivity. Appl Biochem Eng Biotechnol 38:53–86
Kwan GJ, Kuga S, Hori K, Yatagai M, Ando K, Hattori N (2006) Saccharification of cellulose by dry pyrolysis. J Wood Sci 52:461–465
Hosoya T, Kawamoto H, Saka S (2007) Influence of inorganic matter on wood pyrolysis at gasification temperature. J Wood Sci 53:351–357
Yamazaki J, Minami E, Saka A (2006) Liquefaction of beech wood in various supercritical alcohols. J Wood Sci 52:527–532
Seddon KR (1997) Ionic liquids for clean technology. J Chem Tech Biotechnol 68:351–356
Mcewen BA, Ngo LH, Lecompte K, Goldman LJ (1999) Electrochemical properties of imidazolium salt electrochemical capacitor application. J Electrochem Soci 146:1687–1695
Zhang J, Zhang J, Lin L, Chen T, Zhang J, Liu S, Li Z, Ouyang P (2009) Dissolution of microcrystalline cellulose in phosphoric acid-molecular changes and kinetics. Molecules 4:5027–5041
Kafrawy EA (1982) Investigation of the cellulose/LiCl/Dimethylacetamide and Cellulose/LiC1/N-Methyl-2-Pyrrolidinone Solutions by 13C NMR Spectroscopy. J Appl Polym Sci 27:435–2443
Isogai A, Onabe F, Usuda M (1992) Swelling behavior of cellulose by chemical and mechanical treatments. Sen’iGakkai 48:487–492
Potthast A, Rohrling J, Rosenau T, Borgards A, Sixta H, Kosma P (2003) A novel method for the determination of carbonyl groups in cellulosics by fluorescence labeling 3: monitoring oxidative processes. Biomacromolecules 4:743–749
Swatloski RP, Spear SK, Horbrey JD, Rogers RD (2004) Dissolution of cellulose with ionic liquids. J Am Chem Soc 124:4947–4975
Vitz J, Erdmenger T, Haensch C, Schubert US (2009) Extended dissolution studies of cellulose in imidazolium based ionic liquids. Green Chem 11:417–424
Le KA, Sesocousse R, Budtova T (2012) Influence of water on cellulose-EMIMAc solution properties: a viscometric study. Cellulose 19:45–54
Gazit OM, Katz A (2012) Dialkylimidazolium ionic liquids hydrolyze cellulose under mild conditions. Chem Sus Chem 5:1542–1548
Mazza M, Catana DA, Vaca-Garcia C, Cecutti C (2009) Influence of water on the dissolution of cellulose in selected ionic liquids. Cellulose 16:207–215
Erdmenger T, Haensch C, Hoogenboom R, Schubert US (2007) Homogeneous tritylation of cellulose in 1-butyl-3-methylimidazolium chloride. Macromol Biosci 7:440–445
Zhao B, Greiner L, Leitner W (2012) Cellulose solubilities in carboxylate-based ionic liquids. RSC Adv 2:2476–2479
Kosan B, Michels C, Meister F (2008) Dissolution and forming of cellulose with ionic liquids. Cellulose 15:59–66
Remsing RC, Swatloski RP, Rogers RD, Moyna G (2006) Mechanism of cellulose dissolution in the ionic liquid 1-n-butyl-3-methylimidazolium chloride: a 13C and 35/37Cl NMR relaxation study on model systems. Chem Commun 28:1271–1273
Youngs TGA, Hardacre C, Holbrey JD (2007) Glucose solvation by ionic liquid 1,3-methylimidazolium chloride: a simulation study. J Phys Chem 111:13765–13774
Zhang J, Zhang H, Wu J, He J, Xiang J (2010) NMR spectroscopic studies of cellobiose solvation in EmimAc aimed to understand the dissolution mechanism of cellulose in ionic liquids. Phys Chem Phys 12:1941–1947
Liu H, Sale KL, Holmes BM, Simmons BA, Singh S (2010) Understanding the interactions of cellulose with ionic liquids: a molecular dynamics study. J Phys Chem B 114:4293–4301
Xu H, Pan W, Wang R, Zhang D, Liu C (2012) Understanding the mechanism of cellulose dissolution in 1-butyl-3-methylimidazolium chloride ionic liquid via quantum chemistry calculations and molecular dynamics simulations. J Comput Aided Mol Des 26:329–337
Dadi AP, Varanasi S, Schell CA (2006) Enhancement of cellulose saccharification kinetics using an ionic liquid pretreatment step. Biotechnol Bioeng 95:904–910
Lee SH, Doherty TV, Linhardt RJ, Dordick JS (2009) Ionic liquid-mediated selective extraction of lignin from wood leading to enhanced enzymatic cellulose hydrolysis. Biotechnol Bioeng 102:1368–1376
Zhao H, Jones CL, Baker GA, Xia S, Olubajo O, Person VN (2009) Regenerating cellulose from ionic liquids for an accelerated enzymatic hydrolysis. J Biotech 139:47–54
Zhang H, Wu J, Zhang J, He J (2005) 1-Allyl-3-methylimidazolium chloride room temperature ionic liquid: a new and powerful nonderivatizing solvent for cellulose. Macromolecules 38:8272–8277
Cheng G, Varanasi P, Li C, Liu H, Melnichenko YB, Simmons BA, Kent MS, Singh S (2011) Transition of cellulose crystalline structure and surface morphology of biomass as function of ionic liquid pretreatment and its relation to enzymatic. Biomacromolecules 12:933–941
Lu L, Zhangbi L, Xiao Y, Zhenzhen W, Shuxun C (2009) A novel cellulose hydrogel prepared from its ionic liquid solution. Chin Sci Bull 54:1622–1625
Ohno E, Miyafuji H (2013) Reaction behavior of cellulose in an ionic liquid, 1-ethyl-3-methylimidazolium chloride. J Wood Sci 59:221–228
Wu J, Zhang J, Zhang H, He J, Ren Q, Guo M (2004) Homogeneous acetylation of cellulose in a new ionic liquid. Biomacromolecules 5:266–268
Cao Y, Wu J, Meng T, Zhang J, He J, Li H, Zhang Y (2007) Acetone-soluble cellulose acetates prepared by one-step homogeneous acetylation of cornhusk cellulose in an ionic liquid 1-allyl-3-methylimidazolium chloride (AmimCl). Carbohydr Polym 69:665–672
Heinze T, Schwikal K, Barthel S (2005) Ionic liquids as reaction medium in cellulose functionalization. Macromol Biosci 5:520–525
Liu CF, Sun RC, Zhang AP, Ren JL, Wang XA, Qin MH, Cho ZN, Luo W (2007) Homogeneous modification of sugarcane bagasse cellulose with succinic anhydride using a ionic liquid as reaction medium. Carbonhydo Res 342:919–926
Binder JB, Raines RT (2009) Simple chemical transformation of lignocellulosic biomass into furans for fuels and chemicals. J Am Chem Soc 131:1979–1985
Lima S, Neves P, Antunes M, Pillinger M, Ignatyev N, Valente A (2009) Conversion of mono/di/polysaccharides into furan compounds using 1-alkyl-3-methylimidazolium ionic liquids. Appl Catal A 363:93–99
Hsu WH, Lee YY, Peng WH, Wu KCW (2011) Cellulosic conversion in ionic liquids (ILs): effects of H2O/cellulose molar ratios, temperatures, times, and different ILs on the production of monosaccharides and 5-hydroxymethylfurfural (HMF). Catal Today 174:65–69
Qi X, Watanabe M, Aida TM, Smith RL (2011) Catalytic conversion of cellulose into 5-hydroxymethylfurfural in high yields via a two-step process. Cellulose 18:1327–1333
Ignatyev IA, Doorslaer CV, Mertens PGN, Binnemans K, Vos DED (2010) Reductive splitting of cellulose in the ionic liquid 1-butyl-3-methylimidazolium chloride. Chem Sus Chem 3:91–96
Acknowledgments
The authors wish to thank Ms. Chihiro Kosaka, Kyoto Municipal Institute of Industrial Technology and Culture, for her assistance in GC–MS analyses. This research was partly supported by a Grant-in-Aid for Scientific Research (C) (25450246), for which the authors are grateful.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Ohno, E., Miyafuji, H. Decomposition of cellulose in an ionic liquid, 1-ethyl-3-methylimidazolium chloride. J Wood Sci 60, 428–437 (2014). https://doi.org/10.1007/s10086-014-1421-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10086-014-1421-3