Abstract
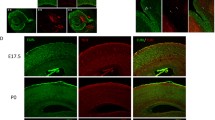
CDKL3 has an important role in regulating cell growth and/or differentiation, and its inactivation is recently reported to be related to non-syndromic mild mental retardation (MR). MR is a common neurological disorder, predominantly characterized by impaired cognitive function. Though genetic factors play a very important role in the pathogenesis of MR, to date, only few genes linked to MR have been characterized and understood very well. Here, we investigated the role of the CDKL3 in the proliferation of cells surrounding the brain ventricle, and the results showed down-regulating CDKL3 by the method of RNAi in the cells surrounding the brain ventricle of the mouse embryo at E15 may inhibit their proliferation. As our previous study had shown that Cdkl3 mRNA expression is developmentally regulated in the central nervous system, peaking during late embryonic and early postnatal stages which are the key stages of neurite formation and maturation, furtherly, the present findings indicated that CDKL3 may be involved in proliferation of cells surrounding the brain ventricle where neuronal progenitor cells are enriched during the late embryo stage, supporting the notion that CDKL3 inactivation contributes to non-syndromic mild MR.


Similar content being viewed by others
References
Yee KW, Moore SJ, Midmer M et al (2003) NKIAMRE, a novel conserved CDC2-related kinase with features of both mitogen-activated protein kinases and cyclin-dependent kinases. Biochem Biophys Res Commun 308:784–792
Dubos A, Pannetier S, Hanauer A (2008) Inactivation of the CDKL3 gene at 5q31.1 by a balanced t (X; 5) translocation associated with nonspecific mild mental retardation. Am J Med Genet A 146A:1267–1279
Aicardi J (1998) The etiology of developmental delay. Semin Pediatr Neurol 5:15–20
Daily DK, Ardinger HH, Holmes GE (2000) Identification and evaluation of mental retardation. Am Fam Phys 61:1059–1067
World Health Organization (2001) The world health report 2001. Mental health: new understanding. WHO Press, New Hope
McLaren J, Bryson SE (1987) Review of recent epidemiological studies of mental retardation: prevalence, associated disorders, and etiology. Am J Ment Retard 92:243–254
Stevenson RE, Schwartz CE, Schroer RL (2000) X-linked mental retardation. Oxford University Press, New York
Mornet E, Bogyo A, Deluchat C et al (1993) Molecular analysis of a ring chromosome X in a family with fragile X syndrome. Hum Genet 92:373–378
Chahrour M, Zoghbi HY (2007) The story of Rett syndrome: from clinic to neurobiology. Neuron 56:422–437
Molinari F, Rio M, Meskenaite V et al (2002) Truncating neurotrypsin mutation in autosomal recessive nonsyndromic mental retardation. Science 298:1779–1781
Higgins JJ, Pucilowska J, Lombardi RQ et al (2004) A mutation in a novel ATP-dependent Lon protease gene in a kindred with mild mental retardation. Neurology 63:1927–1931
Basel-Vanagaite L, Attia R, Yahav M et al (2006) The CC2D1A, a member of a new gene family with C2 domains, is involved in autosomal recessive non-syndromic mental retardation. J Med Genet 43:203–210
Ropers HH (2006) X-linked mental retardation: many genes for a complex disorder. Curr Opin Genet Dev 16:260–269
Saito T, Nakatsuji N (2001) Efficient gene transfer into the embryonic mouse brain using in vivo electroporation. Dev Biol 240:237–246
Liu Z, Xu D, Zhao Y, Zheng J (2010) Non-syndromic mild mental retardation candidate gene CDKL3 regulates neuronal morphogenesis. Neurobiol Dis 39:242–251
Caviness VS Jr, Takahashi T, Nowakowski RS (1995) Numbers, time and neocortical neuronogenesis: a general developmental and evolutionary model. Trends Neurosci 18:379–383
Luo Y, Shan G, Guo W et al (2010) Fragile × mental retardation protein regulates proliferation and differentiation of adult neural stem/progenitor cells. PLoS Genet 6:e1000898
Ming GL, Song H (2005) Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci 28:223–250
Aimone JB, Wiles J, Gage FH (2006) Potential role for adult neurogenesis in the encoding of time in new memories. Nat Neurosci 9:723–727
Doetsch F, Hen R (2005) Young and excitable: the function of new neurons in the adult mammalian brain. Curr Opin Neurobiol 15:121–128
Kaufmann WE, Moser HW (2000) Dendritic anomalies in disorders associated with mental retardation. Cereb Cortex 10:981–991
Moser HW (1995) A role for gene therapy in mental retardation. MRDD Res Rev 1:4–6
Takashima S, Becker LE, Armstrong DL et al (1981) Abnormal neuronal development in the visual cortex of the human fetus and infant with Down’s syndrome. A quantitative and qualitative Golgi study. Brain Res 225:1–21
Takashima S, Ieshima A, Nakamura H et al (1989) Dendrites, dementia and the Down syndrome. Brain Dev 11:131–133
Armstrong D, Dunn JK, Antalffy B et al (1995) Selective dendritic alterations in the cortex of Rett syndrome. J Neuropathol Exp Neurol 54:195–201
Chakrabarti L, Galdzicki Z, Haydar TF (2007) Defects in embryonic neurogenesis and initial synapse formation in the forebrain of the Ts65Dn mouse model of Down syndrome. J Neurosci 27:11483–11495
Contestabile A, Fila T, Ceccarelli C, Bonasoni P, Bonapace L, Santini D, Bartesaghi R, Ciani E (2007) Cell cycle alteration and decreased cell proliferation in the hippocampal dentate gyrus and in the neocortical germinal matrix of fetuses with down syndrome and in Ts65Dn mice. Hippocampus 17:665–678
Escorihuela RM, Vallina IF, Martínez-Cué C, Baamonde C, Dierssen M, Tobeña A, Flórez J, Fernández-Teruel A (1998) Impaired short- and long-term memory in Ts65Dn mice, a model for Down syndrome. Neurosci Lett 247:171–174
Lorenzi HA, Reeves RH (2006) Hippocampal hypocellularity in the Ts65Dn mouse originates early in development. Brain Res 1104:153–159
Callan MA, Cabernard C, Heck J et al (2010) Fragile X protein controls neural stem cell proliferation in the Drosophila brain. Hum Mol Genet 19:3068–3079
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, Z., Tao, D. Inactivition of CDKL3 mildly inhibits proliferation of cells at VZ/SVZ in brain. Neurol Sci 36, 297–302 (2015). https://doi.org/10.1007/s10072-014-1952-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-014-1952-9