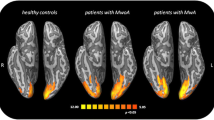
Abstract
Migraine is a neurologic disorder characterized by disabling attacks of throbbing headache with specific features and associated symptoms. Despite the recent discoveries in basic neurosciences, migraine pathophysiology is not completely understood. Nevertheless, in the last decades, advances in functional magnetic resonance imaging (fMRI) have significantly provided new insights into migraine mechanisms. Blood oxygen level dependent (BOLD) fMRI technique is the most commonly used method to explore brain function and connectivity due to high temporal and spatial resolution. The purpose of this review is to present a synthesis of recent BOLD-fMRI studies which have allowed us to elucidate the complex process involved in migraine pathophysiology.
Similar content being viewed by others
Introduction
Migraine is the most prevalent neurologic disorder [1] due to recurrent episodes of cerebral disturbance, often influenced by genetic factors and lifestyle [2], clinically characterized by disabling attacks of throbbing headache with specific features and associated symptoms [3]. Despite the recent discoveries in basic neurosciences, migraine pathophysiology is not completely understood and is a matter of ongoing research. However, in the last decades, advances in non-conventional neuroimaging techniques have significantly provided new insights into migraine mechanisms [4, 5].
Because historical nuclear medicine studies [6, 7], a plethora of functional magnetic resonance (fMRI) studies has been performed to investigate the neural circuitry involved into the pathogenesis of migraine [8]. The most commonly used method among fMRI techniques is the measurement of blood oxygen level dependent (BOLD) signal, based on the differences between magnetic characteristics of oxy-hemoglobin (diamagnetic) and deoxy-hemoglobin (paramagnetic).In the brain, neuronal activity increases consistently with blood flow and oxy-hemoglobin and could be hence visualized by changes in the BOLD contrast. This method enables to explore brain function and connectivity with high temporal and spatial resolution [9]. Two major types of experimental designs utilized in BOLD-fMRI studies are block and event-related designs. Block design experiments are characterized by blocks of identical trial types to establish a task-specific condition. On the other hand, in event-related experiments BOLD-fMRI response is modeled as the linear summation of the hemodynamic response to discrete events [10]. It is well known that migraine episodes are typically unpredictable while BOLD-fMRI studies require considerable planning and this justify the scarcity of fMRI data during spontaneous attacks of migraine [11]. In consequence, BOLD-fMRI research field has been dominated by event-related experiments, specifically triggered migraine attack or experimental acute-pain studies, during interictal period. These BOLD-fMRI studies, although have provided several insight into migraine neuronal mechanisms, are afflicted by the bias of region of interest (ROI) approach, which induce to explore brain function in specific cerebral regions by ‘‘a priori’’ hypothesis. This makes this approach inappropriate to explore whole-brain functional changes in cerebral networks, likely involved in migraine pathophysiology [12]. Nevertheless, more recently, fMRI in the absence of experimental tasks and behavioral responses, performed with the patient in a relaxed “resting” state (RS-fMRI), has allowed for the exploration of brain connectivity between functionally linked cortical regions. Among some different procedures already established and available from BOLD-fMRI, the seed approach [13] is the simplest to investigate spatial patterns, based on the direct correlations with time courses of signal change from a seed measurement. This technique is widely used in functional connectivity mainly due to its ease of interpretation and good sensitivity, however, its main limitation is the dependence on the a priori definition of a seed region, which prevents the method from studying multiple systems simultaneously. To overcome this limitation, blind source separation algorithms, such as independent component analysis (ICA), have become popular in functional connectivity analysis of BOLD-fMRI data. Indeed, independent component analysis (ICA) transforms individual patient RS-fMRI data sets into series of resting-state networks (RSN) maps, allowing for a voxel-based population analysis of whole-brain functional connectivity without the need to specify the ROI constituting the layout of the neural network [14]. The most commonly reported RSN are the default mode network (DMN), the FPN (or executive network), the sensorimotor network and the visual and auditory networks [15].
The purpose of this review is to present an overview of BOLD-fMRI findings that have led to a better understanding of migraine pathophysiology.
Discussion
BOLD-fMRI studies have provided several insights into central “migraine generating” loci, brain network involved in pain processing and cortical spreading depression (CSD) phenomenon in both migraine subtypes (migraine with aura—MwA and migraine without aura—MwoA) [5]. These studies have been performed during migraine aura, ictal headache, and interictal period. Migraine aura remains one of the most intriguing topics in neurology, traditionally accounted as a distinct phase of the migraine attack and connected to the (CSD) phenomenon. Because its original extensive description by Leao in 1944 [16] many fundamental questions regarding its initiation, propagation, functional consequences, and relationship to migraine remain still unanswered [17]. Cao and colleagues [18] have investigated, for the first time by BOLD-fMRI, occipital cortex activation in visually triggered migraine attacks, showing that visual aura was preceded by suppression of activation, related to vasodilatation, slowly propagating into contiguous occipital cortex. Subsequently, these data were confirmed by the same research group although a BOLD signal increase was demonstrated in occipital cortex following red nucleus and substantia nigra activation [19]. These findings suggest that vasodilatation may be associated with the early phase of migraine attack and that brainstem nuclei could be considered as part of a neuronal network activated during a migraine attack. In the same years, Hadjikhani and colleagues [20] have shown that in MwA attacks BOLD signal increase originates in the extrastriate cortex (area V3A), is time locked and consistent with visual progression of a typical migraine aura. Further studies have supported that abnormal cortical excitability, likely due to a lower activation threshold of visual cortical and extra-striate areas [21–24], may represent an important pathophysiological mechanism in migraine aura. Subsequently, there has been a shift towards the study of “functional abnormalities” occurring between attacks both in MwA and MwoA. For example, interictal cortical response has been evaluated by means of different visual stimuli in MwA and MwoA patients confirming a tight connection between cortical hyperexcitability and migraine aura [25]. In the last years, many BOLD-fMRI studies have been performed using experimental pain stimulation to explore pain processing-related cerebral activity in migraine. These studies have shown a widespread subcortical and cortical brain network involved in pain processing in migraine subjects. However, one of the main challenges in the interpretation of these results is to differentiate findings consistent with a general pain response from those that might be specific to migraine [11, 12]. Nevertheless, a noxious stimulation paradigm, using a contact thermode [26], has been extensively used in different BOLD-fMRI studies to elucidate mechanisms underlying pain processing in migraine subjects. Moulton and colleagues [27] have measured brainstem fMRI responses to noxious heat in migraine subjects, showing a reduced activity at the level of the nucleus cuneiformis, a component of brainstem pain modulatory circuits. After this work, similar experiments have been performed in migraine subjects and the same research group has shown an increased BOLD response of the anterior temporal pole in migraine subjects during the interictal period [28]. Similarly, during a moderate noxious stimulus we have demonstrated a significantly greater activation in the anterior cingulate cortex (ACC) and a significantly lower activation in the bilateral somatosensory cortex (SCC) during a severe noxious stimulus, in MwoA patients during interictal period compared to healthy controls (HC) [29]. In a subsequent study [30], in response to different noxious stimuli, we found a region in the pons showing divergent activation between migraine subjects and HC. Taken together, BOLD-fMRI data suggest a compensatory functional reorganization of pain processing network aimed at modulating pain perception. The hypothesis of a compensatory reorganization of anti-nociceptive network in migraine is supported by another BOLD-fMRI study, using repetitive trigeminal–nociceptive stimulation, which showed significant increased ACC and decreased SSC responses in MwoA patients [31]. The same authors reported a cycling behavior of the spinal trigeminal nuclei activity in response to nociceptive stimulation, demonstrating that the trigeminal activation level increases over the pain-free migraine interval [32]. More recently, Stankewitz and May [33] have investigated neuronal substrates of olfactory stimulation during migraine attack and interictal period and, at a later stage, using both trigemino-nociceptive and olfactory stimuli, explored neuronal correlates of habituation in migraine subjects [34]. In the first study, during spontaneous and untreated attacks, in response to olfactory stimulation migraine subjects showed a significantly increased activation in limbic structures and in the rostral pons compared to HC. In the other study, pain and olfactory ratings exhibited divergent behavior in patients and HC, related to bilateral anterior insula, middle cingulate cortex and thalamus activity which was increased in migraine subjects but decreased in HC. These data have highlighted a strong physiologic relationship between the olfactory and the trigemino-nociceptive pathways in the pathophysiology of migraine and have clarified that impaired habituation in functional brain systems is fundamental only to specific modalities (e.g. trigemino-nociceptive stimulation). More recently, RS-fMRI has been applied in studies focused on migraine, to assess alterations of baseline intrinsic brain activity, likely related to long-term migraine attacks. Along this research line, Mainero and colleagues [35] have analyzed the alteration of baseline functional interaction within the periaqueductal gray matter networks. Yu and colleagues [36] have applied regional homogeneity method to analyze local temporal homogeneity of intrinsic fluctuation, and investigated the functional connectivity alterations of regions showing morphometric deficits during rest condition. In a recent RS-fMRI study, we have demonstrated a decreased functional connectivity of FPN, known to be associated with executive functions, in MwoA patients without significant neuropsychological executive deficits [37]. These findings revealed that the observed FPN reduced connectivity may underlie daily living difficulties commonly reported by patients experiencing migraine.
Studies to evaluate both structural and functional cortical measures was used by several authors [35–40], highlighting the impact of enduring migraine pain over brain function and contributing to the idea that long-term and high-frequency headache attacks may cause both structural and functional connectivity network reorganization. Furthermore, RS-fMRI findings may provide specific insights into compensatory functional reorganization mechanisms of the migrainous brain aimed at modulating both pain perception intensity and emotional/cognitive reaction to pain [39, 40]. In conclusion, BOLD-fMRI studies have improved our knowledge of migraine mechanisms underlying migraine aura and neuronal activation during migrainous and experimental pain processing in migraine patients. On the other hand, further studies are needed to better elucidate the neural correlates of different phases of migraine attack to clarify the complexity of migraine pathophysiology.
References
Lipton RB, Bigal ME, Diamond M et al (2007) Migraine prevalence, disease burden, and the need for preventive therapy. Neurology 68(5):343–349
Goadsby PJ, Charbit AR, Andreou AP et al (2009) Neurobiology of migraine. Neuroscience 161(2):327–341
Rasmussen BK, Stewart WF (2000) Epidemiology of migraine. In: Olesen J, Tfelt-Hansen P, Welch KMA (eds) The Headaches, 2nd edn. Lippincott Williams & Wilkins, Philadelphia, pp 228–232
May A (2007) Neuroimaging: visualising the brain in pain. Neurol Sci 28:S101–S107
Lakhan SE, Avramut M, Tepper SJ (2012) Structural and functional neuroimaging in migraine: insights from 3 decades of research. Headache 53(1):46–66
Olesen J, Larsen B, Lauritzen M (1981) Focal hyperemia followed by spreading oligemia and impaired activation of rCBF in classic migraine. Ann Neurol 9(4):344–352
Weiller C, May A, Limmroth V et al (1995) Brain stem activation in spontaneous human migraine attacks. Nat Med 1(7):658–660
May A (2006) A review of diagnostic and functional imaging in headache. J Headache Pain 7(4):174–184
Ogawa S, Lee TM, Kay AR et al (1990) Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci USA 87(24):9868–9872
Petersen SE, Dubis JW (2012) The mixed block/event-related design. Neuroimage 62(2):1177–1184
Schwedt TJ, Dodick DW (2009) Advanced neuroimaging of migraine. Lancet Neurol. 8(6):560–568
Russo A, Tessitore A, Giordano A et al (2012) The pain in migraine beyond the pain of migraine. Neurol Sci 33(Suppl 1):S103–S106
Schöpf V, Kasess CH, Lanzenberger R et al (2010) Fully exploratory network ICA (FENICA) on resting-state fMRI data. J Neurosci Methods 192(2):207–213
Tedeschi G, Esposito F (2012) Neuronal networks observed with resting state functional magnetic resonance imaging in clinical populations. In: Bright P (ed) Neuroimaging-cognitive and clinical neuroscience. InTech. doi:10.5772/23290. ISBN:978-953-51-0606-7
Mantini D, Perrucci MG, Del Gratta C et al (2007) Electrophysiological signatures of resting state networks in the human brain. Proc Natl Acad Sci USA 104(32):13170–13175
Leao AAP (1944) Spreading depression of activity in cerebral cortex. J Neurophysiol 7:359–390
Charles A, Brennan K (2009) Cortical spreading depression-new insights and persistent questions. Cephalalgia 29(10):1115–1124
Cao Y, Welch KM, Aurora S, Vikingstad EM (1999) Functional MRI-BOLD of visually triggered headache in patients with migraine. Arch Neurol 56(5):548–554
Cao Y, Aurora SK, Nagesh V et al (2002) Functional MRI-BOLD of brainstem structures during visually triggered migraine. Neurology 59(1):72–78
Hadjikhani N, Sanchez Del Rio M, Wu O et al (2001) Mechanisms of migraine aura revealed by functional MRI in human visual cortex. Proc Natl Acad Sci USA 98(8):4687–4692
Bramanti P, Grugno R, Vitetta A et al (2005) Migraine with and without aura: electrophysiological and functional neuroimaging evidence. Funct Neurol 20(1):29–32
Vincent M, Pedra E, Mourão-Miranda J et al (2003) Enhanced interictal responsiveness of the migrainous visual cortex to incongruent bar stimulation: a functional MRI visual activation study. Cephalalgia 23(9):860–868
Coutts LV, Cooper CE, Elwell CE, Wilkins AJ (2012) Time course of the haemodynamic response to visual stimulation in migraine, measured using near-infrared spectroscopy. Cephalalgia 32(8):621–629
Antal A, Polania R, Saller K et al (2011) Differential activation of the middle-temporal complex to visual stimulation in migraineurs. Cephalalgia 31(3):338–345
Datta R, Aguirre GK, Hu S, et al (2013) Interictal cortical hyperresponsiveness in migraine is directly related to the presence of aura. Cephalalgia [Epub ahead of print]
Chen AC, Niddam DM, Arendt-Nielsen L (2001) Contact heat evoked potentials as a valid means to study nociceptive pathways in human subjects. Neurosci Lett 316(2):79–82
Moulton EA, Burstein R, Tully S et al (2008) Interictal dysfunction of a brainstem descending modulatory center in migraine patients. PLoS One 3(11):e3799
Moulton EA, Becerra L, Maleki N et al (2011) Painful heat reveals hyperexcitability of the temporal pole in interictal and ictal migraine States. Cereb Cortex 21(2):435–448
Tessitore A, Russo A, Esposito F et al (2011) Interictal cortical reorganization in episodic migraine without aura: an event-related fMRI study during parametric trigeminal nociceptive stimulation. Neurol Sci 32(Suppl 1):S165–S167
Russo A, Tessitore A, Esposito F et al (2012) Pain processing in patients with migraine: an event-related fMRI study during trigeminal nociceptive stimulation. J Neurol 259(9):1903–1912
Aderjan D, Stankewitz A, May A (2010) Neuronal mechanisms during repetitive trigemino-nociceptive stimulation in migraine patients. Pain 151(1):97–103
Stankewitz A, Aderjan D, Eippert F, May A (2011) Trigeminal nociceptive transmission in migraineurs predicts migraine attacks. J Neurosci 31(6):1937–1943
Stankewitz A, May A (2011) Increased limbic and brainstem activity during migraine attacks following olfactory stimulation. Neurology 77(5):476–482
Stankewitz A, Schulz E, May A (2012) Neuronal correlates of impaired habituation in response to repeated trigemino-nociceptive but not to olfactory input in migraineurs: an fMRI study. Cephalalgia 33(4):256–265
Mainero C, Boshyan J, Hadjikhani N (2011) Altered functional magnetic resonance imaging resting-state connectivity in periaqueductal gray networks in migraine. Ann Neurol 70(5):838–845
Yu DH, Yuan K, Zhao L et al (2012) Regional homogeneity abnormalities in patients with interictal migraine without aura: a resting-state study. NMR Biomed 25(5):806–812
Russo A, Tessitore A, Giordano A et al (2012) Executive resting-state network connectivity in migraine without aura. Cephalalgia 32(14):1041–1048
Jin C, Yuan K, Zhao L et al (2012) Structural and functional abnormalities in migraine patients without aura. NMR Biomed 26(1):58–64
Maleki N, Becerra L, Brawn J et al (2012) Concurrent functional and structural cortical alterations in migraine. Cephalalgia 32(8):607–620
Liu J, Zhao L, Li G et al (2012) Hierarchical alteration of brain structural and functional networks in female migraine sufferers. PLoS One 7(12):e51250
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tedeschi, G., Russo, A., Conte, F. et al. The role of BOLD-fMRI in elucidating migraine pathophysiology. Neurol Sci 34 (Suppl 1), 47–50 (2013). https://doi.org/10.1007/s10072-013-1383-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-013-1383-z