Abstract
We present a case of pneumococcal meningitis complicated by leukoencephalopathy. Possible pathogenic mechanisms, a diagnostic pitfall, and optimal treatment options are discussed.
Similar content being viewed by others
Introduction
Pneumococcal meningitis is the most common and the most severe bacterial meningitis seen in adults. Despite the availability of modern antibiotic therapy and advances in critical care medicine, pneumococcal meningitis continues to carry a high risk of mortality and neurologic sequellae [1–3].
Intracranial complications are common, particularly in asplenic patients and patients with comorbidities [4]. Inflammatory host response and direct bacterial cytotoxic action in pneumococcal meningitis lead to irreversible damage of neural tissue [5, 6]. While the systemic complications of bacterial meningitis are readily recognizable and well known, the intracranial complications may be more subtle or even asymptomatic. Furthermore, the spectrum of possible intracranial complications continues to grow, due in part to increased detection of central nervous system abnormalities with magnetic resonance imaging (MRI) [4, 7–10].
Here, we report a case of severe pneumococcal meningitis with leukoencephalopathy and discuss possible pathogenic mechanisms and optimal treatment options.
Case report
A 68-year-old white female was admitted to this hospital because of a 2-day history of fever, severe headache and rapid neurological deterioration. She had a 10-year history of arterial hypertension. Her previous medical history was otherwise unremarkable.
At admission she was febrile and comatose with shallow and irregular respirations. Neurologic examination revealed nuchal rigidity, conjugate eye deviation to the left, and symmetric attenuation of deep tendon reflexes. Immediate intubation and mechanical ventilation were required.
Lumbar puncture at admission revealed 986 white cells per cubic millimeter with 86% polymorphonuclear cells. CSF glucose was 0.0 mmol/L, the total protein concentration was 6.7 g/L, lactate 12.7 mmol/L and chloride 113 mmol/L. Gram staining revealed Gram-positive diplococci.
The peripheral white blood cell count was 7.6 × 109/L, platelet count 138 × 109/L, and hemoglobin level 12.6 g/dL. C-reactive protein was 257 mg/L and erythrocyte sedimentation rate 25 mm/h.
Penicillin-sensitive Streptococcus pneumoniae was cultured from blood and cerebrospinal fluid. PCR for S. pneumoniae from CSF was also positive.
Chest radiography and electrocardiogram showed no abnormalities. An electroencephalogram detected marked diffuse background slowing. A brain CT scan at admission was unremarkable.
Adjuvant steroid treatment followed by antibiotic was started immediately at admission after the lumbar puncture was done. The initial treatment consisted of intravenous ceftriaxone 2 g twice a day followed by 24 million units of benzylpenicillin per day for 14 days and dexamethasone 12 mg every 6 h for the first 4 days.
During the following week, the CSF findings improved but no clinical improvement was noted. On day 7, the second brain CT scan revealed symmetric white matter hypodensities in confluent pattern throughout the brain. Transcranial Doppler (TCD) ultrasound showed significantly decreased mean blood flow velocities (MBFV) and increased pulsatility index (PI) at both middle cerebral arteries (MCA) (31 cm/s and 1.8, respectively). Carbon dioxide reactivity (CO2 R) using the breath holding method was impaired (breath holding index was 0.9. Normal values 1.03–1.65) [11]. The breath holding was performed by disconnection from the ventilator for 30 s. The treatment strategy during this time consisted of volume resuscitation with isotonic saline, low-dose epinephrine pressure support, hemodilution (hypervolemic hypertensive hemodilution therapy) and anticoagulation with heparin for a week.
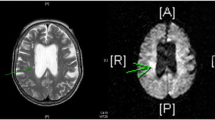
On day 15, MR scan on T2-weighted images and FLAIR (Fluid Attenuation Inversion Recovery) sequence revealed a hyperintense subcortical intracerebral hematoma in the right frontal lobe, with a hypointense rim inside the region of perilesional edema representing a late subacute hemorrhage. Broad regions of high-intensity abnormalities were seen in the white matter of the frontal–parietal–occipital regions. There was a lack of mass effect or enhancement (Fig. 1, Fig. 2).
On day 15, a very slow neurological recovery began. One month later the patient was alert and was able to carry out simple commands. Brain CT scan confirmed resolution of the lesions.
On discharge from the department, the GCS was 13, GOS was 3 and Karnofsky performance score was 40%. Two and a half months after the admission, the patient died suddenly at another hospital ward probably because of a pulmonary embolism. Unfortunately, an MRI of the brain after recovery was not done. The patient’s family refused a postmortem examination.
Discussion
We have described a case of pneumococcal meningitis with unusual white matter changes in an immunocompetent patient. The treatment was appropriate but delayed (48 h from onset of overt signs of meningitis) and an unfavorable disease course complicated by leukoencephalopathy followed. Delay in appropriate treatment related to the illness onset was the most important factor associated with unfavorable outcome [3].
Neuroimaging (CT and MRI of the brain) revealed extensive and symmetric white matter changes without mass effect or enhancement suggestive of demyelination. Faced with the treatment decision difficulties, we concluded that it would be quite unusual for postinfectious demyelination to develop in a patient with bacterial meningitis particularly because of prior dexamethasone adjunctive treatment. In addition, the TCD findings in our patient (decreased MBFV and increased PI with impaired CO2 reactivity) indicated significant cerebral hypoperfusion and impaired microvascular chemoregulation consistent with small vessel vasculitis.
The opportunity to confirm that the finding with jugular bulb oxymetry (SjO2) and lactate-oxygen index (LOI) measurement in our patient was unfortunately missed. Nevertheless, based on transcranial Doppler findings, we presumed that the white matter changes were secondary to severe hypoperfusion due to vasculitis and cytotoxic edema rather than demyelination.
The treatment approach aimed to increase cerebral perfusion pressure with subsequent clinical and neuroradiologic improvement. The new-onset cerebral hematoma was attributed to secondary hemorrhage in areas of ischemia.
While MRI is an important part of the diagnostic assessment, it must be supplemented with additional measurements to guide diagnosis and treatment. Measuring cerebral blood flow (CBF) and hemodynamics can provide critical information about cerebral oxygenation. The jugular bulb catheter should be placed so that the paired blood samples could be collected [arterial and venous (BVJ)]. Desaturation of jugular bulb venous blood (SjO2 < 55%) with increased cerebral lactate-oxygen index (LOI > 0.08) [derived from arterio-jugular venous oxygen content difference (AjVDO2) and arterio-jugular venous lactate concentration difference (AVDL)] are reliable markers of cerebral hypoperfusion if cerebral metabolic oxygen demand (CMRO2) is constant [12–14].
Transcranial Doppler (TCD) ultrasound can reveal cerebral hemodynamic changes in real time by calculating mean blood flow velocities (MBFV) and pulsatility index (PI). In addition, the carbon dioxide reactivity (CO2 R) of the cerebral small arteries and arterioles provides an excellent insight into the cerebral microvascular hemodynamics and blood flow autoregulatory status [15, 16].
MRI white matter changes in bacterial meningitis have been previously reported [7–9]. Jorens et al. [7, 8] described the clinical course, diagnostic work-up, and treatment in four pneumococcal meningoencephalitis cases (three adult cases and one in an infant) with multifocal and diffuse white matter changes. All the patients presented with abrupt onset, low-grade pleocytosis and met criteria of fulminant meningitis. The low-grade pleocytosis accompanied by parenchymal lesions in these patients reflect the spreading of bacteria into the brain tissue and development of necrotizing vasculitis with widespread cytotoxic edema. Even though high-dose methylprednisolone therapy was initiated late in the course of the disease, treatment with steroids resulted in clinical improvement and all of the patients survived. These results are notable because the outcome of fulminant pneumococcal meningoencephalitis is otherwise typically fatal. Beneficial steroid effects in these patients could be due to recovery of blood–brain barrier integrity, resolution of cytotoxic edema and attenuation of immune-mediated vasculitis. These examples suggest that appropriate use of steroids in selected patients could be life saving.
Conclusions
Even in the case of an unremarkable brain CT scan, cerebral vasculitis and cytotoxic edema should be considered in every patient with severe bacterial meningitis, particularly if the period of unconsciousness lasts longer than 3 days. In such cases, the use of MRI together with TCD and jugular bulb oxymetry could significantly help in the assessment of intracranial meningitis related complications and proper treatment decision-making. Diffusion-weighted MRI (DWI) should be always provided in similar cases in order to distinguish ischemic from demyelinating changes.
The reported case confirms the importance of early diagnosis and urgent start of an appropriate antibiotic and corticosteroid therapy. The duration and dosage of steroid adjuvant treatment should be guided by clinical response and may continue after antibiotic therapy is initiated.
References
Durand ML, Calderwood SB, Weber DJ et al (1993) Acute bacterial meningitis in adults. A review of 493 episodes. N Engl J Med 328:21–28
Van de Beek D, de Gans J, Tunkel AR, Wijdicks EFM (2006) Community-acquired bacterial meningitis in adults. N Engl J Med 354:44–53
Lepur D, Baršić B (2007) Community-acquired bacterial meningitis in adults: antibiotic timing in disease course and outcome. Infection 35(4):225–231
Kastenbauer S, Pfister HW (2003) Pneumococcal meningitis in adults: spectrum of complications and prognostic factors in a series of 87 cases. Brain 126(Pt 5):1015–1025
Weber JR, Tuomanen EI (2007) Cellular damage in bacterial meningitis: an interplay of bacterial and host driven toxicity. J Neuroimmunol 184(1–2):45–52
Scheld WM, Koedel U, Nathan B, Pfister HW (2002) Pathophysiology of bacterial meningitis: mechanism(s) of neuronal injury. J Infect Dis 186(Suppl 2):S225–S233
Jorens PG, Parizel PM, Wojciechowski M et al (2008) Streptococcus pneumoniae meningoencephalitis with unusual and widespread white matter lesions. Eur J Paediatr Neurol 12(2):127–132
Jorens PG, Parizel PM, Demey HE et al (2005) Meningoencephalitis caused by Streptococcus pneumoniae: a diagnostic and therapeutic challenge. Diagnosis with diffusion-weighted MRI leading to treatment with corticosteroids. Neuroradiology 47(10):758–764
Abe M, Takayama Y, Yamashita H, Noguchi M, Sagoh T (2002) Purulent meningitis with unusual diffusion-weighted MRI findings. Eur J Radiol 44(1):1–4
Maschke M, Kastrup O, Forsting M, Diener C (2004) Update on neuroimaging in infectious central nervous system disease. Curr Opin Neurol 17(4):475–480
Zavoreo I, Demarin V (2004) Breath holding index in the evaluation of cerebral vasoreactivity. Acta Clin Croat 43:15–19
Macmillan CSA, Andrews PJD (2000) Cerebrovenous oxyen saturation monitoring: practical considerations and clinical relevance. Intensive Care Med 26:1028–1036
Robertson CS, Narayan RK, Gokaslan ZL et al (1989) Cerebral arteriovenous oxygen difference as an estimate of cerebral blood flow in comatose patients. J Neurosurg 70:222–230
Valadka AB, Furuya Y, Hlatky R, Robertson CS (2000) Global and regional techniques for monitoring cerebral oxidative metabolism after severe traumatic brain injury. Neurosurg Focus 9(5):1–3
Babikian VL, Feldman E et al (2000) Transcranial Doppler ultrasonography; year 2000 update. J Neuroimaging 10:101–115
Cigada M, Marzorati S, Tredici S, Iapichino G (2000) Cerebral CO2 vasoreactivity evaluation by transcranial Doppler ultrasound technique: a standardized methodology. Intensive Care Med 26:729–732
Acknowledgments
We thank Ms Ilana Richman, Ms Arijana Pavelić and Mrs Andrea Deduš for their help in the preparation of the manuscript.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lepur, D., Peterković, V., Višković, K. et al. Leukoencephalopathy in pneumococcal meningitis: a diagnostic pitfall and treatment challenge. Neurol Sci 32, 139–142 (2011). https://doi.org/10.1007/s10072-010-0336-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-010-0336-z