Abstract
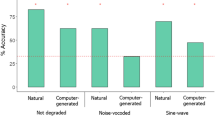
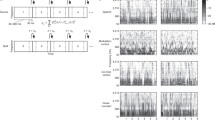
Previous research demonstrated that a language-trained chimpanzee recognized familiar English words in sine-wave and noise-vocoded forms (Heimbauer et al. Curr Biol 21:1210–1214, 2011). However, those results did not provide information regarding processing strategies of the specific acoustic cues to which the chimpanzee may have attended. The current experiments tested this chimpanzee and adult humans using sine-wave and noise-vocoded speech manipulated using specific sine-waves and a different number of noise bands, respectively. Similar to humans tested with the same stimuli, the chimpanzee was more successful identifying sine-wave speech when both SW1 and SW2 were present – the components that are modeled on formants F1 and F2 in the natural speech signal. Results with noise-vocoded speech revealed that the chimpanzee and humans performed best with stimuli that included four or five noise bands, as compared to those with three and two. Overall, amplitude and frequency modulation over time were important for identification of sine-wave and noise-vocoded speech, with further evidence that a nonhuman primate is capable of using top-down processes for speech perception when the signal is altered and incomplete.





Similar content being viewed by others
References
Beran MJ (2010) Use of exclusion by a chimpanzee (Pan troglodytes) during speech perception and auditory-visual matching-to-sample. Behav Process 83:287–291
Beran MJ, Heimbauer LA (2015) A longitudinal assessment of vocabulary retention in symbol-competent chimpanzees (Pan troglodytes). PLoS ONE 10:e0118408. https://doi.org/10.1371/journal.pone.0118408
Beran MJ, Washburn DA (2002) Chimpanzee responding during matching to sample: Control by exclusion. J Exp Anal Behav 78:497–508
Beran MJ, Pate JL, Washburn DA, Rumbaugh DM (2004) Sequential responding and planning in chimpanzees (Pan troglodytes) and rhesus macaques (Macaca mulatta). J Exp Psychol Anim Behav Process 30:203–212
Boersma P, Weenink D (2008) Praat: doing phonetics by computer [Computer program]. Version 5.1.11. http://www.praat.org/. Retrieved 1 Sept 2008
Brakke KE, Savage-Rumbaugh ES (1995a) The development of language skills in bonobo and chimpanzee–I. Comprehens Lang Commun 15:121–148
Brakke KE, Savage-Rumbaugh ES (1995b) The development of language skills Pan-II. Prod Lang Commun 16:361–380
Davis MH, Johnsrude IS (2007) Hearing speech sounds: Top-down influences on the interface between audition and speech perception. Hear Res 229:132–147
Davis MH, Johnsrude IS, Hervais-Adelman A, Taylor K, McGettigan C (2005) Lexical information drives perceptual learning of distorted speech: Evidence from the comprehension of noise-vocoded sentences. J Exp Psychol Gen 134:222–241
Dorman MF, Loizou PC, Spahr AJ, Maloff E (2002) A comparison of the speech understanding provided by acoustic models of fixed-channel and channel-picking signal processors for cochlear implants. J Speech Lang Hear R 45:783–788
Drullman R (2006) The significance of temporal modulation frequencies for speech intelligi-bility. In: Greenberg S, Ainsworth WA (eds) Listening to speech: An auditory perspective. Erlbaum, NJ, pp 43–67
Greenwood DD (1961) Critical bandwidth and the frequency coordinates of the basilar membrane. J Acoust Soc Am 33:1344–1356
Greenwood DD (1990) A cochlear frequency-position function for several species – 29 years later. J Acoust Soc Am 87:2592–2605
Heimbauer LA, Beran MJ, Owren MJ (2011) A chimpanzee recognizes synthetic speech with significantly reduced acoustic cues to phonetic content. Curr Biol 21:1210–1214
Heimbauer LA, Beran MJ, Owren MJ (2018) A chimpanzee’s (Pan troglodytes) perception of variations in speech: Identification of familiar words when whispered and when spoken by a variety of talkers. Int J Psychol 31:1–16
Hillenbrand JM, Clark MJ, Baer CA (2011) Perception of sinewave vowels. J Acoust Soc Am 129:3991–4000
Kluender KR, Diehl RL, Killeen PR (1987) Japanese quail can learn phonetic categories. Science 237:1195–1197
Kuhl PK (1988) Auditory perception and the evolution of speech. Hum Evol 3:19–43
Kuhl PK, Miller JD (1975) Speech perception by the chinchilla: Voiced-voiceless distinction in alveolar plosive consonants. Science 190:69–72
Kuhl PK, Padden DM (1982) Enhanced discriminability at the phonetic boundaries for the voicing feature in macaques. Percept Psychophys 35:542–550
Kuhl PK, Padden DM (1983) Enhanced discriminability at the phonetic boundaries for the place feature in macaques. J Acoust Soc Am 73:1003–1010
Lenneberg EH (1967) Biological foundations of language. Wiley, NY
Lewis DE, Carrell TD (2007) The effect of amplitude modulation on intelligibility of time-varying sinusoidal speech in children and adults. Percept Psychophys 69:1140–1151
Liberman AM (1982) On finding that speech is special. Am Psychol 37:148–167
Mann VA, Liberman AM (1983) Some differences between phonetic and auditory modes of perception. Cognition 14:211–235
Marcus G, Vijayan S, Rao S, Vishton PM (1999) Rule learning by seven-month-old infants. Science 283:77–80
Newman RS (2006) Perceptual restoration in toddlers. Percept Psychophys 68:625–642
Nygaard LC, Pisoni DB (1998) Talker specific learning in speech perception. Percept Psychophys 60:355–376
Nygaard LC, Sommers MS, Pisoni DB (1994) Speech perception as a talker-contingent process. Psychol Sci 5:42–46
Owren MJ (2010) GSU Praat Tools: Scripts for modifying and analyzing sounds using Praat acoustics software. Behav Res Methods 40:822–829
Pisoni DB (1995). Some thought on "normalization" in speech perception. Research on Spoken Language Processing, Progress Report No. 20, Indiana University 3–29.
Remez RE (2005) Perceptual organization of speech. In: Pisoni DB, Remez RE (eds) The handbook of speech perception. Wiley-Blackwell, Oxford, pp 28–50
Remez RE, Rubin PE (1990) On the perception of speech from time-varying acoustic information: Contributions of amplitude variation. Percept Psychophy 48:313–325
Remez RE, Rubin PE, Pisoni DB, Carrell TD (1981) Speech perception without traditional speech cues. Science 212:947–949
Remez RE, Rubin PE, Berns SM, Lang PJS, JM, (1994) On the perceptional organization of speech. Psychol Rev 101:129–156
Remez RE, Dubowski KR, Broder RS, Davids ML, Grossman YS, Moskalenko M, Pardo JS, Hasbun SM (2011) Auditory-phonetic projection and lexical structure in the recognition of sine-wave words. J Exp Psychol Human 37:968–977
Remez RE, Thomas EF, Dubowski KR, Koinis SM, Porter NAC, Paddu NU, Moskalenko M, Grossman YS (2013) Modulation sensitivity in the perceptual organization of speech. Atten Percept 75:1353–1358
Rosner BS, Talcott JB, Witton C, Hogg JD, Richardson AJ, Hansen PC, Stein JF (2003) The perception of “Sine-Wave Speech” by adults with developmental dyslexia. J Speech Lang Hear Res 46(1):68–79
Rumbaugh DM, Washburn DA (2003) Intelligence of apes and other rational beings. Yale University, CT
Saffran JR, Aslin RN, Newport EL (1996) Statistical learning by 8 month-old infants. Science 274:1926–1928
Sawusch JR (2005) Acoustic analysis and synthesis of speech. In: Pisoni DB, Remez RE (eds) The handbook of speech perception. Blackwell, Oxford, pp 7–27
Shannon RV, Zeng F, Kamath V, Wygonski J, Ekelid M (1995) Speech recognition with primarily temporal cues. Science 270:303–304
Sommers MS, Kirk KI, Pisoni DB (1997) Some considerations in evaluating spoken word recognition by normal-hearing, noise-masked normal-hearing, and cochlear implant listeners. I. The effects of response format. Ear Hear 18:89–99
Souza P, Rosen S (2009) Effects of envelope bandwidth on the intelligibility of sine- and noise-vocoded speech. J Acoust Soc Am 126:792–805
Trout JD (2001) The biological basis of speech: What to infer from talking to the animals. Psychol Rev 108:523–549
Werker JF, Desjardins RN (1995) Listening to speech in the first year of life: Experiential influences on phoneme perception. Curr Dir Psychol Sci 4:76–81
Whalen DH, Liberman AM (1987) Speech perception takes precedence over nonspeech perception. Science 237:169–171
Acknowledgements
We thank the care staff at Georgia State University for their care of Panzee throughout this experiment and the College of Arts and Sciences at Georgia State University for financial support for this project.
Funding
Work was supported by the National Institute of Child Health and Human Development, National Science Foundation, GSU’s RCALL and Brains & Behavior programs, the Center for Behavioral Neuroscience under the Science and Technology Centers Program of the National Science Foundation under agreement IBN-9876754, and ERC grant agreement AdG249516. LAH was funded by an RCALL Fellowship and a Duane M. Rumbaugh Fellowship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no competing interests and no conflict of interest.
Availability of data and material
The data are available in the Open Science Framework.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Michael J. Owren deceased (2014).
Rights and permissions
About this article
Cite this article
Heimbauer, L.A., Beran, M.J. & Owren, M.J. A chimpanzee recognizes varied acoustical versions of sine-wave and noise-vocoded speech. Anim Cogn 24, 843–854 (2021). https://doi.org/10.1007/s10071-021-01478-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10071-021-01478-4