Abstract
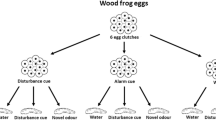
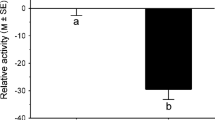
Recognition of predation risk from cues released from injured heterospecific could be beneficial when prey belongs to the same prey guild. Here, we performed three experiments. Experiment 1 showed that P. thaul tadpoles reduced their activity levels when exposed to conspecific injury cues, but not when exposed to amphipod injury cues. Experiment 2 tested whether P. thaul tadpoles can learn to recognize predation risk from chemical cues released from injured heterospecifics from the same prey guild (amphipod, Hyalella patagonica). A group of tadpoles were conditioned by exposing them to a specific concentration of amphipod injury cues paired with conspecific injury cues. Two days later, we evaluated changes in the activity of tadpoles when they were exposed to amphipod cues. As a control of learning, we used an unpaired group. Additionally, we used more control groups to fully investigate the learning mechanism. Our results showed that tadpoles can learn to recognize predation risk from injured amphipods and that the mechanism underlying the observed learned response could be associative. Experiment 3 replicated Experiment 2 and also showed that a low concentration of amphipod cues did not sustain that learning.



Similar content being viewed by others
References
Avarguès-Weber A, Dawson EH, Chittka L (2013) Mechanisms of social learning across species boundaries. J Zool 290(1):1–11
Batabyal A, Gosavi SM, Gramapurohit NP (2014) Determining sensitive stages for learning to detect predators in larval bronzed frogs: importance of alarm cues in learning. J Biosci 39(4):701–710
Brown GE, Ferrari MCO, Elvidge CK, Ramnarine I, Chivers DP (2013) Phenotypically-plastic neophobia: a response to variable predation risk. Proc R Soc Lond B 280(1756):20122712
Chivers DP, Smith RJF (1998) Chemical alarm signaling in aquatic predator/prey interactions: a review and prospectus. Ecoscience 5(3):338–352
Chivers DP, Wisenden BD, Smith RJF (1995) The role of experience in the response of fathead minnows (Pimephales promelas) to skin extract of Iowa darters (Etheostoma exile). Behaviour 132(9–10):665–674
Chivers DP, Mirza RS, Johnston J (2002) Learned recognition of heterospecific alarm cues enhances survival during encounters with predators. Behaviour 139(7):929–938
Crane AL, Lampe MJ, Mathis A (2013) Detecting danger from prey-guild members: behavioural and metabolic responses of Ozark zigzag salamanders to alarm secretions from earthworms. Ethol Ecol Evol 25(4):377–387
Domjan M (2003) Principios de aprendizaje y conducta. Thomson, Madrid
Dukas R (2002) Behavioural and ecological consequences of limited attention. Philos Trans R Soc Lond B Biol Sci 357(1427):1539–1547
Dukas R (2013) Effects of learning on evolution: robustness, innovation and speciation. Anim Behav 85(5):1023–1030
Ferrari MCO, Kapitania-Kwok T, Chivers DP (2006) The role of learning in the development of threat-sensitive predator avoidance: the use of predator cue concentration by fathead minnows. Behav Ecol Sociobiol 60(4):522–527
Ferrari MCO, Brown GE, Messier F, Chivers DP (2009) Threat-sensitive generalization of predator recognition by amphibians. Behav Ecol Sociobiol 63(9):1369–1375
Ferrari MCO, Wisenden BD, Chivers DP (2010a) Chemical ecology of predator-prey interactions in aquatic ecosystems: a review and prospectus. Can J Zool 88(7):698–724
Ferrari MCO, Brown GE, Bortolotti GR, Chivers DP (2010b) Linking predator risk and uncertainty to adaptive forgetting: a theoretical framework and empirical test using tadpoles. Proc R Soc B 277(1691):2205–2210
Gonzalo A, López P, Martín J (2010) Risk level of chemical cues determines retention of recognition of new predators in Iberian green frog tadpoles. Behav Ecol Sociobiol 64(7):1117–1123
Gosner KL (1960) A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica 16(3):183–190
Gregory PT (2013) Once bitten, twice shy: does previous experience influence behavioural decisions of snakes in encounters with predators? Ethology 119(11):1–7
Hermann SL, Thaler JS (2014) Prey perception of predation risk: volatile chemical cues mediate non-consumptive effects of a predator on a herbivorous insect. Oecologia 176(3):669–676
Jara FG (2014) Trophic ontogenetic shifts of the dragonfly Rhionaeschna variegata: the role of larvae as predators and prey in Andean wetland communities. Ann Limnol-Int J Limnol 50(2):173–184
Jara FG, Perotti MG (2010) Risk of predation and behavioural response in three anuran species: influence of tadpole size and predator type. Hydrobiologia 644(1):313–324
Jara FG, Úbeda CA, Perotti MG (2013) Predatory insects in lentic freshwater habitats from northwest Patagonia: richness and phenology. J Nat Hist 47(43–44):2749–2768
Jayne K, Lea SEG, Leaver LA (2015) Behavioural responses of Eastern grey squirrels, Sciurus carolinensis, to cues of risk while foraging. Behav Process 116:53–61
Lima SL, Dill LM (1990) Behavioral decisions made under the risk of predation: a review and prospectus. Can J Zool 68(4):619–640
Martín J, Ortega J, López P (2015) Experience may allow increasing accuracy of the innate chemosensory recognition of snake predators by Iberian wall lizards. Behav Ecol Sociobiol 69(9):1565–1572
Mathis A, Ferrari MCO, Windel N, Messier F, Chivers DP (2008) Learning by embryos and the ghost of predation future. Proc R Soc B 275(1651):2603–2607
Mirza RS, Chivers DP (2000) Predator-recognition training enhances survival of brook trout: evidence from laboratory and field enclosure studies. Can J Zool 78(12):2198–2208
Mirza RS, Chivers DP (2001) Learned recognition of heterospecific alarm signals: the importance of a mixed predator diet. Ethology 107(11):1007–1018
Mirza RS, Chivers DP (2003) Fathead minnows learn to recognize heterospecific alarm cues they detect in the diet of a known predator. Behaviour 140(11):1359–1369
Mirza RS, Ferrari MCO, Kiesecker JM, Chivers DP (2006) Responses of American toad tadpoles to predation cues: behavioural response thresholds, threat-sensitivity and acquired predation recognition. Behaviour 143(7):887–889
Nelson AB, Alemadi SD, Wisenden BD (2013) Learned recognition of novel predator odour by convict cichlid embryos. Behav Ecol Sociobiol 67(8):1269–1273
Pollock MS, Chivers DP (2004) The effects of density on the learned recognition of heterospecific alarm cues. Ethology 110(5):341–349
Pollock MS, Chivers DP, Mirza RS, Wisenden BD (2003) Fathead minnows, Pimephales promelas, learn to recognize chemical alarm cues of introduced brook stickleback, Culaea inconstans. Environ Biol Fish 66(4):313–319
Polo-Cavia N, Gomez-Mestre I (2014) Learned recognition of introduced predators determines survival of tadpole prey. Funct Ecol 28(2):432–439
Pueta M, Cruz FB, Perotti MG (2016) Feeding regime and food availability determine behavioural decisions under predation risk in Pleurodema thaul (Anura: Leiuperidae) tadpoles. Herpetol J 26(1):61–64
Rochette R, Arsenault DJ, Justome B, Himmelman JH (1998) Chemically-mediated predator-recognition learning in a marine gastropod. EcoScience 5(3):353–360
Ryan KM, Blumstein DT, Blaisdell AP, Stahlman WD (2012) Stimulus concordance and risk-assessment in hermit crabs (Coenobita clypeatus): implications for attention. Behav Process 91:26–29
Turner AM, Turner SE, Lappi HM (2006) Learning, memory and predator avoidance by freshwater snails: effects of experience on predator recognition and defensive strategy. Anim Behav 72(6):1443–1450
Wisenden BD, Chivers DP (2006) The role of public chemical information in antipredator behaviour. In: Ladich F, Collins SP, Moller P, Kapoor BG (eds) Fish communication. Science Publisher, NH, pp 259–278
Wisenden BD, Millard MC (2001) Aquatic flatworms use chemical cues from injured conspecifics to assess predation risk and to associate risk with novel cues. Anim Behav 62(4):761–766
Wisenden BD, Chivers DP, Smith RJF (1997) Learned recognition of predation risk by Enallagma damselfly larvae (Odonata, Zygoptera) on the basis of chemical cues. J Chem Ecol 23(1):137–151
Wisenden BD, Cline A, Sparkes TC (1999) Survival benefit to antipredator behavior in the amphipod Gammarus minus (Crustacea:Amphipoda) in response to injury-released chemical cues from conspecifics and heterospecifics. Ethology 105(5):407–414
Acknowledgments
We are grateful to P Abate, C Arias, the editor in chief and the two anonymous reviewers, who provided helpful comments to improve this work. This work was supported by Agencia Nacional de Promoción Científica y Tecnológica (PICT-BID 13-2384 to MP), Consejo Nacional de Investigaciones Científicas y Técnicas (PIP-11220110100782 to MGP) and Universidad Nacional del Comahue (UNCo B 166), Argentina.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standard
Experiments were performed under the Guidelines for Use of Live Amphibians and Reptiles in Field Research (ASIH). Experiments followed the ethical norms imposed by Argentina (APN N° 1231). Samplings in Laguna Fantasma were authorized by Secretaria de Medio Ambiente of San Carlos de Bariloche, Río Negro, Argentina (Licence N° 41-AP-2014).
Rights and permissions
About this article
Cite this article
Pueta, M., Perotti, M.G. Anuran tadpoles learn to recognize injury cues from members of the same prey guild. Anim Cogn 19, 745–751 (2016). https://doi.org/10.1007/s10071-016-0971-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10071-016-0971-8