Abstract
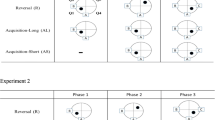
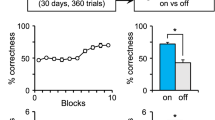
Behavioral work has demonstrated that rats solve many spatial problems using a conditional strategy based on orientation at the start point. The present study assessed whether mice use a similar strategy and whether the strategy would be affected by the poorer directional sensitivity of mice. In Experiment 1, mice were trained on a response, a direction or one of two place problems to locate a hidden platform in a water T-maze located in two positions. In the response task, mice made a right (or left) turn from two different start points located 180° apart. In the direction task, the maze was shifted (to the left or right) and the start points rotated by 180° across trials, but the platform was in a constant direction relative to room cues. In the translation place task, the mice were trained to locate the platform in a fixed location relative to extra-maze cues when the maze was shifted across trials, but the orientation of the start arm did not change. In the rotation place task, the mice were trained to locate the platform in a fixed location when the maze was shifted and the start points rotated by 90° across trials. As previously reported with rats, mice had difficulty solving the translation place problem compared with the other three problems. Unlike rats, mice learned the direction problem in significantly fewer trials than the rotation problem. This difference between acquisition of the direction and rotation problems was replicated in Experiment 2. The difficulty mice have in discriminating start point orientations that are 90° apart as opposed to 180° apart can be attributed to the broader firing ranges of HD cells in mice compared with rats.




Similar content being viewed by others
References
Blodgett HC, McCutchen K, Mathews R (1949) Spatial learning in the t-maze: the influence of direction, turn, and food location. J Exp Psychol 39:800–809
Brown RE, Wong AA (2007) The influence of visual ability on learning and memory performance in 13 strains of mice. Learn Mem 14:134–144
Cheung A, Sturzl W, Zeil J, Cheng K (2008) The information content of panoramic images II: view-based navigation in nonrectangular experimental arenas. J Exp Psychol Anim Behav Proc 34:15–30
Dudchenko PA, Zinyuk LE (2005) The formation of cognitive maps of adjacent environments: evidence from the head direction cell system. Behav Neurosci 119:1511–1523
Dwyer JA, Ingram ML, Snow AC, Thorpe CT, Martin GM, Skinner DM (2013) The effects of bilateral lesions to the dorsal tegmental nucleus on spatial learning in rats. Behav Neurosci 127:867–877
Frick KM, Stillner ET, Berger-Sweeney J (2000) Mice are not little rats: species differences in a one-day water maze task. NeuroReport 11:3461–3465
Fyhn M, Molden S, Witter MP, Moser EI, Moser M (2004) Spatial representation in the entorhinal cortex. Science 305:1258–1264
Fyhn M, Hafting T, Witter MP, Moser EI, Moser M (2008) Grid cells in mice. Hippo 18:1230–1238
Hafting T, Fyhn M, Molden S, Moser M, Moser EI (2005) Microstructure of a spatial map in the entorhinal cortex. Nature 436:801–806
Hamilton DA, Akers KG, Weisend MP, Sutherland RJ (2007) How do room and apparatus cues control navigation in the Morris water task? Evidence for distinct contributions to a movement vector. J Exp Psychol Anim Behav Proc 33:100–114
Kentros CG, Agnihotri NT, Streater S, Hawkins RD, Kandel ER (2004) Increased attention to spatial context increases both place field stability and spatial memory. Neuron 42:283–295
Knierim JJ, Hamilton DA (2011) Framing spatial cognition: neural representations of proximal and distal frames of reference and their roles in navigation. Physiol Rev 91:1245–1279
McHugh TJ, Blum KI, Tsien JZ, Tonegawa S, Wilson MA (1996) Impaired hippocampal representation of space in CA1-specific NMDAR1 knockout mice. Cell 87:1339–1349
McNaughton BL, Battaglia FP, Jensen O, Moser EI, Moser M (2006) Path integration and the neural basis of the ‘cognitive map’. Nat Rev Neurosci 7:663–678
Moser EI, Kropff E, Moser M (2008) Place cells, grid cells, and the brain’s spatial representation system. Ann Rev Neurosci 31:69–89
Muller R (1996) A quarter of a century of place cells. Neuron 17:979–990
O’Keefe J, Dostrovsky J (1971) The hippocampus as a spatial map: preliminary evidence from unit activity in the freely-moving rat. Brain Res 34:171–175
O’Leary TP, Brown RE (2012) The effects of apparatus design and test procedure on learning and memory performance of C57BL/6 J mice on the Barnes maze. J Neurosci Meth 203:315–324
O’Leary TP, Savoie V, Brown RE (2011) Learning, memory and search strategies of inbred mouse strains with different visual abilities in the Barnes maze. Behav Brain Res 216:531–542
Passino E, Middei S, Restivo L, Bertaina-Anglade V, Ammassari-Teule M (2002) Genetic approach to variability of memory systems: analysis of place vs. response learning and Fos-related expression in hippocampal and striatal areas of C57BL/6 and DBA/2 mice. Hippo 12:63–75
Peckford G, McRae SM, Thorpe CM, Martin GM, Skinner DM (2013) Rats’ orientation at the start point is important for spatial learning in a water T-maze. Learn Motiv 44:1–15
Prusky GT, West PWR, Douglas RM (2000) Behavioral assessment of visual acuity in mice and rats. Vis Res 40:2201–2209
Rotenberg A, Mayford M, Hawkins RD, Kandel ER, Muller RU (1996) Mice expressing activated CaMKII lack low frequency LTP and do not form stable place cells in the CA1 region of the hippocampus. Cell 87:1351–1361
Sheynikhovich D, Chavarriaga R, Strosslin T, Arleo A, Gerstner W (2009) Is there a geometric module for spatial orientation? Insight from a rodent navigation model. Psy Rev 116:540–566
Skinner DM, Etchegary CM, Ekert-Maret EC, Baker CJ, Harley CW, Evans JH, Martin GM (2003) An analysis of response, direction, and place learning in an open field and T maze. J Exp Psychol Anim Behav Proc 29:3–13
Skinner DM, Horne MR, Murphy KEA, Martin GM (2010) Rats’ orientation is more important than start point location for successful place learning. J Exp Psychol Anim Behav Proc 36:110–116
Stackman RW, Lora JC, Williams SB (2012) Directional responding of C57BL/6 J mice in the Morris water maze is influenced by visual and vestibular cues and is dependent on the anterior thalamic nuclei. J Neurosci 32:10211–10225
Stringer KG, Martin GM, Skinner DM (2005) The effects of hippocampal lesions on response, direction and place learning in rats. Behav Neurosci 119:946–952
Sturzl W, Cheung A, Cheng K, Zeil J (2008) The information content of panoramic images I: the rotational errors and the similarity of views in rectangular experimental arenas. J Exp Psychol Anim Behav Proc 34:1–14
Sung JY, Goo JS, Lee DE, Jin DQ, Bizon JL, Gallagher M, Han JS (2008) Learning strategy selection in the water maze and hippocampal CREB phosphorylation differ in two inbred strains of mice. Learn Mem 15:183–188
Taube JS (2007) The head direction signal: origins and sensory-motor integration. Ann Rev Neurosci 30:181–207
Taube JS, Muller RU, Ranck JB Jr (1990a) Head-direction cells recorded from the postsubiculum in freely moving rats. I Description and quantitative analysis. J Neurosci 10:420–435
Taube JS, Muller RU, Ranck JB Jr (1990b) Head-direction cells recorded from the postsubiculum in freely moving rats. II Effects of environmental manipulations. J Neurosci 10:436–447
Tunur T, Dohanich GP, Schrader LA (2010) Pre-exposure to context affects learning strategy selection in mice. Learn Mem 17:328–331
Wahlsten D, Cooper SF, Crabb JC (2005) Different rankings of inbred mouse strains on the morris maze and a refined 4-arm water escape task. Behav Brain Res 165:36–51
Whishaw IQ (1995) A comparison of rats and mice in a swimming pool place task and matching to place task: some surprising differences. Physiol Behav 58:687–693
Whishaw IQ, Tomie JA (1996) Of mice and mazes: similarities between mice and rats on dry-land but not water mazes. Physiol Behav 60:1191–1197
Whyte JT, Martin GM, Skinner DM (2009) An assessment of response, direction and place learning by rats in a water T-maze. Learn Motiv 40:376–385
Yoder RM, Taube JS (2009) Head direction cell activity in mice: robust directional signal depends on intact otolith organs. J Neurosci 29:1061–1076
Acknowledgments
This work was supported by an NSERC Grant to DMS.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cahill, S.P.A., Fifield, K.E., Thorpe, C.M. et al. Mice use start point orientation to solve spatial problems in a water T-maze. Anim Cogn 18, 195–203 (2015). https://doi.org/10.1007/s10071-014-0789-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10071-014-0789-1