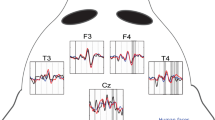
Abstract
Previously, social and cognitive abilities of dogs have been studied within behavioral experiments, but the neural processing underlying the cognitive events remains to be clarified. Here, we employed completely non-invasive scalp-electroencephalography in studying the neural correlates of the visual cognition of dogs. We measured visual event-related potentials (ERPs) of eight dogs while they observed images of dog and human faces presented on a computer screen. The dogs were trained to lie still with positive operant conditioning, and they were neither mechanically restrained nor sedated during the measurements. The ERPs corresponding to early visual processing of dogs were detectable at 75–100 ms from the stimulus onset in individual dogs, and the group-level data of the 8 dogs differed significantly from zero bilaterally at around 75 ms at the most posterior sensors. Additionally, we detected differences between the responses to human and dog faces in the posterior sensors at 75–100 ms and in the anterior sensors at 350–400 ms. To our knowledge, this is the first illustration of completely non-invasively measured visual brain responses both in individual dogs and within a group-level study, using ecologically valid visual stimuli. The results of the present study validate the feasibility of non-invasive ERP measurements in studies with dogs, and the study is expected to pave the way for further neurocognitive studies in dogs.




Similar content being viewed by others
References
Adachi I, Kuwahata H, Fujita K (2007) Dogs recall their owner’s face upon hearing the owner’s voice. Anim Cogn 10:17–21
Allison T, Ginter H, McCarthy G, Nobre AC, Puce A, Luby M, Spencer DD (1994) Face recognition in human extrastriate cortex. J Neurophysiol 71:821–825
Allison T, Puce A, Spencer DD, McCarthy G (1999) Electrophysiological studies of human face perception. I: potentials generated in occipitotemporal cortex by face and non-face stimuli. Cereb Cortex 9:415–430
Autier-Dérian D, Deputte BL, Chalvet-Monfray K, Coulon M, Mounier L (2013) Visual discrimination of species in dogs (Canis familiaris). Anim Cogn. doi:10.1007/s10071-013-0600-8
Avidan G, Harel M, Hendler T, Ben-Bashat D, Zohary E, Malach R (2002) Contrast sensitivity in human visual areas and its relationship to object recognition. J Neurophysiol 87:3102–3116
Berendt M, Hogenhaven H, Flagstad A, Dam M (1999) Electroencephalography in dogs with epilepsy: similarities between human and canine findings. Acta Neurol Scand 99:276–283
Berns GS, Brooks AM, Spivak M (2012) Functional MRI in awake unrestrained dogs. PLoS ONE. doi:10.1371/journal.pone.0038027
Bichsel P, Oliver JE, Coulter DB, Brown J (1988) Recording of visual-evoked potentials in dogs with scalp electrodes. J Vet Intern Med 2:145–149
Bruce V, Young AW (1998) In the eye of the beholder: the science of face perception. University Press, Oxford
Bruce CJ, Desimone R, Gross CG (1981) Visual properties of neurones in a polysensory area in the superior temporal sulcus of the macaque. J Neurophysiol 46:369–384
Carmel D, Bentin S (2002) Domain specificity versus expertise: factors influencing distinct processing of faces. Cognition 83:1–29
Caton R (1875) The electric currents of the brain. Br Med J 2:278
Coles MGH, Rugg MD (1995) Event-related brain potentials: an introduction. In: Rugg MD, Coles MGH (eds) Electrophysiology of mind: event-related brain potentials and cognition, 1st edn. Oxford University Press, New York, pp 1–26
Fukushima H, Hirata S, Ueno A, Matsuda G, Fuwa K, Sugama K, Kusunoki K, Hirai M, Hiraki K, Tomonaga M, Hasegawa T (2010) Neural correlates of face and object perception in an awake chimpanzee (Pan troglodytes) examined by scalp-surface event-related potentials. PLoS ONE. doi:10.1371/journal.pone.0013366
Gardner JL, Sun P, Waggoner RA, Ueno K, Tanaka K, Cheng K (2005) Contrast adaptation and representation in human early visual cortex. Neuron 47:607–620
Gross CG, Rocha-Miranda CE, Bender DB (1972) Visual properties of neurons in inferotemporal cortex of the Macaque. J Neurophysiol 35:96–111
Guo K, Meints K, Hall C, Hall S, Mills D (2009) Left gaze bias in humans, rhesus monkeys and domestic dogs. Anim Cogn 12:409–418
Haider M, Spong P, Lindsley DB (1964) Attention, vigilance, and cortical evoked-potentials in humans. Science 145:180–182
Hänninen L, Mäkelä JP, Rushen J, de Passillé AM, Saloniemi H (2008) Assessing sleep state in calves through electrophysiological and behavioural recordings: a preliminary study. Appl Anim Behav Sci 111:235–250
Hare B, Brown M, Williamson C, Tomasello M (2002) The domestication of social cognition in dogs. Science 298:1634–1636
Haxby JV, Horwitz B, Ungerleider LG, Maisog JM, Pietrini P, Grady CL (1994) The functional organization of human extrastriate cortex: a PET-rCBF study of selective attention to faces and locations. J Neurosci 14:6336–6353
Howell TJ, Conduit R, Toukhsati S, Bennett P (2012) Auditory stimulus discrimination recorded in dogs, as indicated by mismatch negativity (MMN). Behav Process 89:8–13
James FMK, Allen DG, Bersenas AME, Grovum WL, Kerr CL, Monteith G, Parent JM, Poma R (2011) Investigation of the use of three electroencephalographic electrodes for long-term electroencephalographic recording in awake and sedated dogs. Am J Vet Res 72:384–390
Jeserevics J, Viitmaa R, Cizinauskas S, Sainio K, Jokinen TS, Snellman M, Bellino C, Bergamasco L (2007) Electroencephalography findings in healthy and finnish spitz dogs with epilepsy: visual and background quantitative analysis. J Vet Intern Med 21:1299–1306
Kanwisher N, McDermott J, Chun MM (1997) The fusiform face area: a module in human extrastriate cortex specialized for face perception. J Neurosci 17:4302–4311
Kendrick KM (1991) How the sheep’s brain controls the visual recognition of animals and humans. J Anim Sci 69:5008–5016
Kendrick KM (1994) Neurobiological correlates of visual and olfactory recognition in sheep. Behav Process 33:89–112
Kendrick KM, Baldwin BA (1987) Cells in temporal cortex of conscious sheep can respond preferentially to the sight of faces. Science 236:448–450
King AS (1999) Physiological and clinical anatomy of the domestic mammals. Volume 1. Central nervous system. Oxford University Press, Oxford
Koelsch S, Heinke W, Sammler D, Olthoff D (2006) Auditory processing during deep propofol sedation and recovery from unconsciousness. Clin Neurophysiol 117:1746–1759
Kujala MV, Tanskanen T, Parkkonen L, Hari R (2009) Facial expressions of pain modulate observer’s long-latency responses in superior temporal sulcus. Hum Brain Mapp 30:3910–3923
Leopold DA, Rhodes G (2010) A comparative view of face perception. J Comp Psychol 124:233–251
Lopes da Silva FH, van Rotterdam A, Storm van Leeuwen W, Tielen AM (1970a) Dynamic characteristics of visual evoked potentials in the dog. I. Cortical and subcortical potentials evoked by sine wave modulated light. Electroencephalogr Clin Neurophysiol 29:246–259
Lopes da Silva FH, van Rotterdam A, Storm van Leeuwen W, Tielen AM (1970b) Dynamic characteristics of visual evoked potentials in the dog. II. Beta frequency selectivity in evoked potentials and background activity. Electroencephalogr Clin Neurophysiol 29:260–268
Luck SJ (2005) An introduction to the event-related potential technique. The MIT Press, London
Mangun GR (1995) Neural mechanisms of visual selective attention. Psychophysiology 32:4–18
McCarthy G, Puce A, Gore JC, Allison T (1997) Face-specific processing in the human fusiform gyrus. J Cogn Neurosci 9:605–610
McKone E, Kanwisher N, Duchaine BC (2006) Can generic expertise explain special processing for faces? Trends Cogn Sci 11:8–15
Nagasawa M, Murai K, Mogi K, Kikusui T (2011) Dogs can discriminate human smiling faces from blank expressions. Anim Cogn 14:525–533
O’Donnell B, Swearer J, Smith L, Hokama H, McCarley R (1997) A topographic study of ERPs elicited by visual feature discrimination. Brain Topogr 10:133–143
Otten LJ, Rugg MD (2005) Interpreting event-related brain potentials. In: Handy TC (ed) Event-related potentials. A methods handbook. The MIT Press, Cambridge, pp 3–16
Pellegrino FC, Sica REP (2004) Canine electroencephalographic recording technique: findings in normal and epileptic dogs. Clin Neurophysiol 115:477–487
Perrett DI, Rolls ET, Caan W (1982) Visual neurones responsive to faces in the monkey temporal cortex. Exp Brain Res 47:329–342
Perrett DI, Smith PA, Potter DD, Mistlin AJ, Head AS, Milner AD, Jeeves MA (1985) Visual cells in the temporal cortex sensitive to face view and gaze direction. Proc R Soc Lond B 223:293–317
Perrett DI, Mistlin AJ, Chitty AJ, Smith PA, Potter DD, Broennimann R, Harries M (1988) Specialized face processing and hemispheric asymmetry in man and monkey: evidence from single unit and reaction time studies. Behav Brain Res 29:245–258
Pineda JA, Sebestyen G, Nava C (1994) Face recognition as a function of social attention in non-human primates: an ERP study. Cogn Brain Res 2:1–12
Puce A, Allison T, Gore JC, McCarthy G (1995) Face-sensitive regions in human extrastriate cortex studied by functional MRI. J Neurophysiol 74:1192–1199
Racca A, Amadei E, Ligout S, Guo K, Meints K, Mills D (2010) Discrimination of human and dog faces and inversion responses in domestic dogs (Canis familiaris). Anim Cogn 13:525–533
Range F, Aust U, Steurer M, Huber L (2008) Visual categorization of natural stimuli by domestic dogs. Anim Cogn 11:339–347
Rolls ET (1994) Brain mechanisms for invariant visual recognition and learning. Behav Process 33:113–138
Rolls ET, Baylis GC (1986) Size and contrast have only small effects on the responses to faces of neurons in the cortex of the superior temporal sulcus of the monkey. Exp Brain Res 65:38–48
Somppi S, Törnqvist H, Hänninen L, Krause C, Vainio O (2012) Dogs do look at images: eye tracking in canine cognition research. Anim Cogn 15:163–174
Tarr MJ, Cheng YD (2003) Learning to see faces and objects. Trends Cogn Sci 7:23–30
Tate AJ, Fischer H, Leigh AE, Kendrick KM (2006) Behavioural and neurophysiological evidence for face identity and face emotion processing in animals. Philos Trans R Soc B 361:2155–2172
Ternman E, Hänninen L, Pastell M, Agenäs S, Nielsen P (2012) Sleep in dairy cows recorded with a non-invasive EEG technique. Appl Anim Behav Sci 140:25–32
Tsao DY, Freiwald WA, Knutsen TA, Mandeville JB, Tootell RBH (2003) Faces and objects in macaque cerebral cortex. Nat Neurosci 6:989–995
Tsao DY, Freiwald WA, Tootell RBH, Livingstone MS (2006) A cortical region consisting entirely of face-selective cells. Science 311:670–674
Ueno A, Hirata S, Fuwa K, Sugama K, Kusunoki K, Matsuda G, Fukushima H, Hiraki K, Tomonaga M, Hasegawa T (2008) Auditory ERPs to stimulus deviance in an awake chimpanzee (Pan troglodytes): towards hominid cognitive neurosciences. PLoS ONE. doi:10.1371/journal.pone.0001442
Ueno A, Hirata S, Fuwa K, Sugama K, Kusunoki K, Matsuda G, Fukushima H, Hiraki K, Tomonaga M, Hasegawa T (2010) Brain activity in an awake chimpanzee in response to the sound of her own name. Biol Lett 6:311–313
Van der Marel E, Dagnelie G, Spekreijse H (1984) Subdurally recorded pattern and luminance EPs in the alert rhesus monkey. Electroencephalogr Clin Neurophysiol 57:354–368
Vogel EK, Luck SJ (2000) The visual N1 component as an index of a discrimination process. Psychophysiology 37:190–203
Woodman GF (2012) Homologues of human ERP components in nonhuman primates. In: Luck SJ, Kappenman ES (eds) Oxford handbook of event-related potential components, 1st edn. Oxford University Press, New York, pp 611–626
Woodman GF, Kang M-S, Rossi AF, Schall JD (2007) Nonhuman primate event-related potentials indexing covert shifts of attention. Proc Natl Acad Sci USA 104:15111–15116
Acknowledgments
This study was supported by the Academy of Finland (project #137931 to OV, and #115215 and #137511 to CMK), Foundations’ Post-Doc Pool (Kone Foundation), Finnish Cultural Foundation, Advancement of Technology Foundation, Emil Aaltonen Foundation and the BRAHE network (Brain Research collaboration between Aalto University and the University of Helsinki). We thank Timo Murtonen for the custom-made dog chin rest and EEG trigger system; Aino Pikkusaari, Pirkko Nokkala and Martti Siimekselä for the stimulus photos; Mari Palviainen for the help in training of the dogs and conducting the EEG pilot measurements; Tarja Pääkkönen for the advice in the EEG recordings; Mari Vainionpää for the help in the computed tomography acquisition; Antti Flyck and Kristian Törnqvist for the technical support and Katja Irvankoski for the help with Presentation® software.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Törnqvist, H., Kujala, M.V., Somppi, S. et al. Visual event-related potentials of dogs: a non-invasive electroencephalography study. Anim Cogn 16, 973–982 (2013). https://doi.org/10.1007/s10071-013-0630-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10071-013-0630-2