Abstract
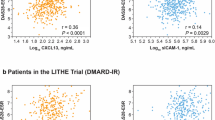
The objective of this study was to compare the effects of treatment by combined conventional disease-modifying antirheumatic drugs (cDMARDs) or biologics on cytokines, disease activity, and function in rheumatoid arthritis (RA). Sera from a cohort of 81 patients with long-standing RA treated with combined cDMARDs or biologics were measured for 12 cytokines. Comparisons of serum cytokine concentrations with treatment types (combination 2, 3 cDMARDs or biologics), serologic status (positivity for RF and anti-cyclic citrullinated peptide antibody (anti-CCP Ab)), DAS28-ESR, and function were performed. Spearman correlation coefficients between individual cytokines and clinical parameters were explored. Approximately half of the patients were prescribed two cDMARDs. Mean duration of current treatment was 42 months. More than 70 % had moderate disease activity or normal function/slight disability. Serum concentrations of interleukin (IL)-1β, IL-2, IL-4, IL-6, IL-8, IL-10, IL-17A, IL-23, IL-33, interferon (IFN)-γ, granulocyte monocyte-colony stimulating factor (GM-CSF), and TNF-α in patients taking combined cDMARDs did not significantly differ from those on biologics. Seventy-nine serum samples (97.5 %) had undetectable levels of 1 to 10 cytokines. Concentrations of several cytokines were significantly higher in patients with moderate to high disease activity, seropositive or poor functional status. Weak correlations between cytokine levels and RA disease activity or function were demonstrated. The highest correlation coefficients were observed with IL-33, IL-8, and IL-6. Long-term treatment with cDMARDs did not differ from biologics with respect to cytokine concentrations, disease activity, and function. The cytokine profiles in established RA were mainly those produced from effector cells, especially IL-6, IL-8, and IL-33. Both IL-8 and IL-33 may be potential biomarkers and/or treatment targets in patients with late RA.


Similar content being viewed by others
References
Choy E (2012) Understanding the dynamics: pathways involved in the pathogenesis of rheumatoid arthritis. Rheumatology (Oxford) 51(Suppl 5):v3–v11
McInnes IB, Schett G (2007) Cytokines in the pathogenesis of rheumatoid arthritis. Nat Rev Immunol 7:429–442
Gizinski AM, Fox DA (2014) T cell subsets and their role in the pathogenesis of rheumatic disease. Curr Opin Rheumatol 26:204–210
McInnes IB, Schett G (2011) The pathogenesis of rheumatoid arthritis. New Engl J Med 365:2205–2219
Davignon JL, Hayder M, Baron M et al (2013) Targeting monocytes/macrophages in the treatment of rheumatoid arthritis. Rheumatology (Oxford) 52:590–598
Furst DE, Emery P (2014) Rheumatoid arthritis pathophysiology: update on emerging cytokine and cytokine-associated cell targets. Rheumatology (Oxford) 53:1560–1569
Okada Y, Wu D, Trynka G et al (2014) Genetics of rheumatoid arthritis contribute to biology and drug discovery. Nature 506:376–381
Alunno A, Manetti M, Caterbi S et al. (2015) Altered immunoregulation in rheumatoid arthritis: the role of regulatory T cells and proinflammatory Th17 cells and therapeutic implications. Mediators Inflam 751793
Smolen JS, Aletaha D, Bijlsma JW et al (2010) Treating rheumatoid arthritis to target: recommendations of an international task force. Ann Rheum Dis 69:631–637
Smolen JS, Breedveld FC, Burmester GR et al (2016) Treating rheumatoid arthritis to target: 2014 update of the recommendations of an international task force. Ann Rheum Dis 75:3–15
Mottonen T, Hannonen P, Leirisalo-Repo M et al (1999) Comparison of combination therapy with single-drug therapy in early rheumatoid arthritis: a randomised trial. FIN-RACo trial group Lancet 353:1568–1573
Grigor C, Capell H, Stirling A et al (2004) Effect of a treatment strategy of tight control for rheumatoid arthritis (the TICORA study): a single-blind randomised controlled trial. Lancet 364:263–269
Goekoop-Ruiterman YP, de Vries-Bouwstra JK, Allaart CF et al (2005) Clinical and radiographic outcomes of four different treatment strategies in patients with early rheumatoid arthritis (the BeSt study): a randomized, controlled trial. Arthritis Rheum 52:3381–3390
Verstappen SM, Jacobs JW, van der Veen MJ et al (2007) Intensive treatment with methotrexate in early rheumatoid arthritis: aiming for remission. Computer Assisted Management in Early Rheumatoid Arthritis (CAMERA, an open-label strategy trial). Ann Rheum Dis 66:1443–1449
Bakker MF, Jacobs JW, Welsing PM et al (2012) Low-dose prednisone inclusion in a methotrexate-based, tight control strategy for early rheumatoid arthritis: a randomized trial. Ann Intern Med 156:329–339
Hirata S, Dirven L, Shen Y et al (2013) A multi-biomarker score measures rheumatoid arthritis disease activity in the BeSt study. Rheumatology (Oxford) 52:1202–1207
Segurado OG, Sasso EH (2014) Vectra DA for the objective measurement of disease activity in patients with rheumatoid arthritis. Clin Exp Rheumatol 32(Suppl 85):S29–S34
Li W, Sasso EH, Emerling D et al (2013) Impact of a multi-biomarker disease activity test on rheumatoid arthritis treatment decisions and therapy use. Curr Med Res Opin 29:85–92
Wu B, Wilson A, Wang F et al (2012) Cost effectiveness of different treatment strategies in the treatment of patients with moderate to severe rheumatoid arthritis in China. PLoS One. doi:10.1371/journal.pone.0047373
Fragoulakis V, Vitsou E, Hernandez AC et al (2015) Economic evaluation of anti-TNF agents for patients with rheumatoid arthritis in Greece. Clinicoecon Outcomes Res 7:85–93
Thai Rheumatism Association (2008) Guideline for biological therapy in rheumatoid arthritis. http://www.thairheumatology.org/download/guideline_biolo_gic_therapy.pdf (Thai language) Accessed 1 December 2015
Arnett FC, Edworthy SM, Bloch DA et al (1988) The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 31:315–324
Osiri M, Wongchinsri J, Ukritchon S et al (2009) Comprehensibility, reliability, validity and responsiveness of the Thai version of the Health Assessment Questionnaire in Thai patients with rheumatoid arthritis. Arthritis Res Ther 11:R129
Bartels EM, Ribel-Madsen S (2013) Cytokine measurements and possible interference from heterophilic antibodies—problems and solutions experienced with rheumatoid factor. Methods 61:18–22
Boers M, Verhoeven AC, Markusse HM et al (1997) Randomised comparison of combined step-down prednisolone, methotrexate and sulphasalazine with sulphasalazine alone in early rheumatoid arthritis. Lancet 350:309–318
O’Dell JR, Mikuls TR, Taylor TH et al (2013) Therapies for active rheumatoid arthritis after methotrexate failure. New Engl J Med 369:307–318
Scott DL, Ibrahim F, Farewell V et al (2015) Therapy with conventional disease modifying anti-rheumatic drugs in established rheumatoid arthritis: TACIT non-inferiority randomised controlled trial. BMJ 350:h1046
Alex P, Szodoray P, Knowlton N et al (2007) Multiplex serum cytokine monitoring as a prognostic tool in rheumatoid arthritis. Clin Exp Rheumatol 25:584–592
Raza K, Falciani F, Curnow SJ et al (2005) Early rheumatoid arthritis is characterized by a distinct and transient synovial fluid cytokine profile of T cell and stronal cell origin. Arthritis Res Ther 7:R784–R795
Hueber W, Tomooka BH, Zhao X et al (2007) Proteomic analysis of secreted proteins in early rheumatoid arthritis: anti-citrulline autoreactivity is associated with up regulation of proinflammatory cytokines. Ann Rheum Dis 66:712–719
Wright HL, Bucknall RC, Moots RJ et al (2012) Analysis of SF and plasma cytokines provides insights into the mechanisms of inflammatory arthritis and may predict response to therapy. Rheumatology (Oxford) 51:451–459
McInnes IB, Buckley CD, Isaacs JD (2016) Cytokines in rheumatoid arthritis-shaping the immunological landscape. Nat Rev Rheumatol 12:163–168
Acknowledgements
We thank Associate Professor Somrat Lertmaharit, Department of Preventive and Social Medicine, Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand, for statistical consultation; Ms. Chaweewan Punkum and Ms. Duangporn Emampaiwong, Bio-Rad Laboratories, Ltd., Bangkok, Thailand, for performing multiplex cytokine assays and technical assistance; and Professor Robert J. Moots, Institute of Ageing and Chronic Disease, University of Liverpool, Liverpool, UK, for scientific consultation and critical review of the manuscript.
Authors’ contributions
MO initiated the concept and design of the study; collected, analyzed, and interpreted the data; prepared the manuscript; and finalized it according to the recommendations. JW initiated the study design, analyzed and interpreted the data, and critically revised the manuscript. YS cleaned the raw data, entered the cleaned data to SPSS program,analyzed the data, and created all of the figures. NT acquired the laboratory data and tested them for accuracy and integrity and interpreted the laboratory data. All of the authors read and approved the entire content of the submitted manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study was approved by Chulalongkorn University Institutional Review Board (study IRB number 533/56).
Funding
This study was funded by Ratchadapiseksompotch Fund, Faculty of Medicine, Chulalongkorn University, grant number RA 57/072. The funding body had no influence on the design, analysis, or results reported of this study.
Disclosures
None.
Rights and permissions
About this article
Cite this article
Osiri, M., Wongpiyabovorn, J., Sattayasomboon, Y. et al. Inflammatory cytokine levels, disease activity, and function of patients with rheumatoid arthritis treated with combined conventional disease-modifying antirheumatic drugs or biologics. Clin Rheumatol 35, 1673–1681 (2016). https://doi.org/10.1007/s10067-016-3306-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-016-3306-x