Abstract
A 32-year-old motorcyclist who was hit by a tram subsequently presented with blunt force thoracic trauma, a pelvic fracture and a penetrating injury to the left lower extremity. Coagulopathy persisted following surgery of the leg and pelvic vascular intervention. Bedside thoracotomy was performed to treat pneumothorax and pneumopericardium. Severe hypoxemia secondary to lung failure ensued, which required venovenous extracorporeal membrane oxygenation (VV ECMO) support. On the third day after the trauma, ultra-protective mechanical ventilation was not possible due to non-existent lung compliance; thus, the ventilator was disconnected, and the T-piece was connected to the blocked tracheal tube left in the airway. Gas exchange occurred via VV ECMO separately. After 48 h of cessation of ventilator support, the patient was weaned from sedation. At this time, respiratory effort was observed, and assisted ventilation was initiated. The patient ultimately recovered and experienced an excellent outcome. The clinical significance of zero end-expiratory pressure (ZEEP) and the complete cessation of open lung strategy during ECMO remains controversial. In cases of reduced lung compliance, if VV ECMO can facilitate adequate gas exchange, the discontinuation of ventilation is an option that can be used to prevent ventilator-induced lung damage and to allow the lungs to rest. VV ECMO is feasible as lung support with no mechanical ventilation in case of severe lung failure after major trauma.
Similar content being viewed by others
Introduction
Severe lung failure is a common complication of major trauma [1]. Prior reports have described case series involving the successful use of venovenous extracorporeal membrane oxygenation (VV ECMO) without heparin in hemorrhagic shock patients [2]. Severe bleeding following multiple traumas is not a contraindication for VV ECMO.
Abdominal compartment syndrome (ACS) is another complication observed in severely ill patients, particularly following abdominal or blunt trauma. ACS results in decreased lung compliance and elevated intra-abdominal pressure, which complicate the use of mechanical ventilation.
Case report
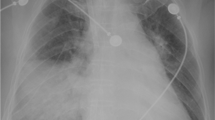
A 32-year-old male motorcyclist was involved in a collision with a tram. In the field, his Glasgow Coma Scale score was 15, his heart rate was 130 beats/min, his blood pressure was 150/90 mmHg, his respiratory rate was 18 breaths/min, and his oxygen saturation was 93%. He was intubated and mechanically ventilated upon arrival at the emergency room. Imaging studies, including abdominal ultrasound, X-rays of the chest and pelvis and a whole-body CT scan, revealed the following injuries [Injury Severity Score (ISS) = 66]:
-
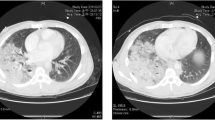
blunt thoracic trauma, serial rib fractures, a right-sided lung contusion, a small left-sided pneumothorax, and a small pneumopericardium;
-
a stable pelvic fracture;
-
a severe penetrating injury to the lower extremity with compartment syndrome, a III–IV degree open fracture of the distal femur, a fracture of the patella, and fractures of the proximal tibia and fibula with injuries to the popliteal artery and vein.
The following procedures were performed after the placement of a chest tube: external fixation of the lower extremity, autologous graft reconstruction of the popliteal artery, and creation of a compartment incision. Diffuse bleeding from the extremities persisted at the time of the patient’s admission to the intensive care unit (ICU). The patient required increasing doses of vasopressors and increased invasivity of mechanical ventilation. Secondary CT scan was performed and revealed worsening pneumopericardium, which was repaired via anterolateral bedside thoracotomy with a ventral pericardial window and pericardial suture placement.
Due to the bleeding and coagulopathy, the patient received a mass transfusion of 38 units of packed red blood cells (PRBCs), 20 units of fresh frozen plasma (FFP), 9 units of platelets, Tranexamic acid 1 mg/kg BW, 16 g of fibrinogen, 4000 IU of prothrombin complex concentrate and 1250 mg of factor XIII within the first 24 h following admission. The indications for VV ECMO support were hypoxemia with an arterial pO2 of 97 mmHg, hypercapnia with a pCO2 of 93 mmHg and a pH of 7.12 secondary to lung failure and decreased lung compliance (tidal volume less than 200 mL although ΔP > 20).
Cannulation for VV ECMO was performed percutaneously via the right jugular vein (with a 21 French cannula) and the right femoral vein (with a 23 French cannula), and both cannulas were coated with heparin. A CardioHelp HLS® System (Maquet, Raststatt, Germany) was used for cannulation, and 5000 IU of heparin was administered during this procedure. No heparin was administered during the first 5 days of VV ECMO run. No parts of the circuit had to be changed due to clots, and no clots were observed during the run.
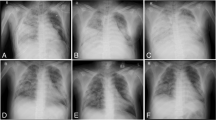
On the third day post-trauma, the patient developed ACS (intraabdominal pressure >30 mm H2O, as measured using a pressure transducer in the urinary bladder, which had been filled with 100 mL of NaCl). Renal function was impaired (oliguric), and lung compliance deteriorated extensively and rapidly within an hour (tidal volume less than 50 mL on ventilator settings with PEEP 15 and inspiratory pressure of 35 cm H2O). Exploratory laparotomy was performed at the patient’s bedside. The renal function had improved, but lung compliance had not. Postoperatively, the ventilator was disconnected, and the endotracheal tube was left blocked in the trachea, with a T-piece placed at the end of the tube. Thus, gas exchange was achieved through VV ECMO alone. During the next 48 h, the patient’s sedation was weaned and respiratory effort (thorax movement) was observed. The spontaneous breaths were sporadic, occurring at a rate less than 10 per minute, and very shallow. Percutaneous dilatational tracheotomy was performed. Subsequently, assisted ventilation with very low tidal volumes (20–30 mL) was initiated. No atelectasis was present. The patient’s lung compliance increased to 300 mL over the following 3 days. The patient was weaned from VV ECMO and mechanical ventilation on days 9 and 13, respectively, after the trauma (Fig. 1). He was discharged from the ICU after 16 days and from the hospital after 49 days. His recovery and outcome after 12 months follow-up revealed no neurological disability.
Discussion
The “lung rest” ventilation approach uses an extremely low VT, such as 1 cc/kg, or pressure-limited ventilation with appropriate PEEP levels during VV ECMO support. Maintaining some level of PEEP might have prevented the additional atelectrauma [3] that this patient incurred based on the management approach reported in this case report. However, the approach reported in this manuscript involves a different strategy.
The initial indication for and true objective of VV ECMO is the maximum possible reduction of high ventilator settings toward the use of only a “lung-protective” ventilator strategy (tidal volume of 4–6 mL/kg ideal body weight) rather than the complete cessation of mechanical ventilation. However, in the described case, when ACS occurred at 1 day after the initiation of VV ECMO, mechanical ventilation was stopped and was reserved for use only as a last-resort therapy. The lungs may have contributed to some oxygenation (apnoic oxygenation and CO2 removal) as X-ray revealed that they remained air filled. Extracorporeal Life Support Organization (ELSO) guidelines suggest a capacity of VV ECMO sufficient to supply all metabolic oxygen requirements in the absence of lung function. In general, VV ECMO is used with additional mechanical ventilation for lung support for critically ill individuals. The oxygenation values achieved by VV ECMO depend on the cannula size and the patient’s height, weight, dynamic recirculation, cardiac output, right ventricle function and pulmonary artery pressure. In case of vast or fully extracorporeal lung support, very low oxygenation parameters can be targeted and tolerated, such as pO2 > 50 mmHg and SaO2 > 75%. In the described case, the use of VV ECMO support allowed the temporary discontinuation of mechanical ventilation and the resting of the lungs. Several research groups have recommended the use of this approach in different contexts. In particular, this approach has been suggested as the basis for the “awake ECMO” concept (e.g., as a bridging strategy and alternative to mechanical ventilation prior to lung transplantation to allow for mobilization and to avoid physical deconditioning) [4, 5]. The novel strategy in the present case involved the use of VV ECMO to allow temporary stoppage of mechanical ventilation, with the patient able to engage in sporadic spontaneous breathing through an endotracheal tube with a T-piece.
Mechanical ventilation can be stopped independent of the underlying pulmonary pathology; however, the cessation of ventilation has potential mechanistic and clinical advantages and risks. During uncontrolled wake up, strong expiration efforts with increasing pressure can occur. Sedation weaning under controlled conditions is recommended to avoid any risk or hazard.
The advantages of the approach described in this case report include a decreased level of sedation and the options of performing tracheotomy and restarting ventilation. The potential risks include the impacts of zero end-expiratory pressure (ZEEP) on ECMO flow and recirculation, diaphragmatic atrophy, impaired bronchial clearance and irreversible atelectasis. Low-pressure ventilation with the possibility to trigger the patient’s own pressure-supported breaths might counteract these complications.
Whether ZEEP is better than a low level of PEEP (e.g., 4–6 cm H2O) remains controversial. Different ventilation strategies are being studied in animal experiments and in various patient groups. In the animal model of Borges with healthy lungs, ZEEP together with low FIO2 promoted heterogeneity of ventilation and lung tissue densities, leading to greater airway closure and ventilation inhomogeneities in poorly aerated regions [6]. Among ICU patients without ALI or ARDS, a strategy of lower tidal volume (VT) + higher positive end-expiratory pressure (PEEP) resulted in the highest PaO2/FIO2 ratio among several investigated strategies. Furthermore, a strategy of higher VT + lower PEEP was superior to the other strategies in improving pulmonary compliance, whereas a strategy of lower VT + lower PEEP was associated with a shorter length of ICU stay; a strategy of lower VT + zero end-expiratory pressure (ZEEP) was associated with the lowest PaO2/FiO2 ratio and pulmonary compliance [7].
High levels of invasivity can be detrimental to the patient. Ventilatory settings can interact with the passive process of expiration and generate intrinsic or auto-PEEP. Adverse effects of PEEP application can occur in patients with auto-PEEP upon an increase in total PEEP [8]. In more than one-third of patients with acute lung injury, alveolar recruitment resulting from positive end-expiratory pressure (PEEP) leads to the over-inflation of previously aerated lung regions [9]. In a comparison of intraoperative strategies, ventilation with high PEEP resulted in the increased production of inflammatory markers [10]. In a review of recent studies that used volume-limited or pressure-limited ventilation in ALI/ARDS patients, higher PEEP was not associated with significantly different short-term mortality or barotrauma. The review does not support the routine use of higher PEEP in patients with ALI/ARDS [11].
Two other approaches have been used routinely with beneficial effects in similar situations. One of these consists of the use a CPAP between 5 and 10 cm H2O with no attempt to achieve any tidal volume in a spontaneously breathing patient. The other involves the use of low-pressure high-frequency oscillation ventilation (HFOV) with a MAP of 13 and low amplitude and ΔP. Both of these approaches avoid ZEEP as well as excessive high airway pressures and allow the patient to breathe as they recover and the possibility of excessive inspiratory effort with high negative trans-pulmonary pressures.
The option was not feasible because the patient was deeply sedated (Ramsay 5) and presented no spontaneous respiratory activity. His condition worsened within a short time of 30 min and we had to act. In such an emergency situation, the only option was to disconnect the ventilation and not to increase the lung damage by over-distending and rupturing alveoli and by triggering a secondary inflammatory response syndrome that intensifies the lung injury. After 48 h, we recorded exactly this option as the patient began to again breathe spontaneously.
High-frequency oscillation ventilation is an alternative type of ventilation used to maintain small tidal volumes that are delivered at high frequencies (3–15 Hz) with an oscillation pump to ensure constant lung recruitment. This form of ventilation satisfies the strategic goal of protective lung ventilation with extremely small tidal volumes (1–4 ml/kg) and constant lung recruitment. However, HFOV therapy has several complications, such as barotrauma and haemodynamic compromise. Another disadvantage of HFOV is that patients require a deep sedation to tolerate this therapy, which yields negative effects. The return to spontaneous physiological breathing and sedation breaks, which appear to be factors in lung protective treatment, is not possible [12]. It is possible that the positive physiological effects and benefits of lung recruitment afforded by HFOV could lead to the continuous regeneration of collapsed and inflamed lung units.
A primary concern involving the described approach is the potential reversibility of lung tissue changes and a lack of benefits from the ZEEP procedure. We achieved sufficient lung recruitment, lung recovery and maintenance of oxygenation. Because the duration of mechanical ventilation cessation was relatively short, irreversible lung collapse, a feared negative effect of ZEEP, did not occur. The physiological state was restored, which was entirely favorable. Irreversibility can occur after longer periods of invasive, unprotective mechanical ventilation that induce VILI and barotrauma. We postulate that the cessation of mechanical ventilation and the allowance of spontaneous breathing were more effective than the use of neuromuscular blockers during the short period of 48 h, allowing non-invasive lung rest. This therapeutic strategy promoted the improvement of alveolar damage because invasive mechanical ventilation, which can have a traumatizing effect on the lung parenchyma and alveoli, was avoided.
The nature of the underlying pulmonary disease was regarded as a combination of a lung contusion, transfusion-related lung injuries after multiple transfusions, high hydrostatic pressure edema caused by hypervolemia and positive fluid balance caused by low peripheral resistance due to posttraumatic systemic inflammatory response syndrome. The overall diagnosis was summarized as severe lung failure requiring venovenous extracorporeal lung support.
Conclusions
The main message is that a patient can be fully oxygenated on VV ECMO without ventilation of the lungs. In cases of poor lung compliance (VT less than 100 mL), if gas exchange can be fully facilitated by VV ECMO, the discontinuation of mechanical ventilation until the return of spontaneous breathing activity might prevent ventilator-induced lung damage and allow the lungs to rest.
References
Michaels AJ. Management of post traumatic respiratory failure. Crit Care Clin. 2004;20:83–99.
Arlt M, Philipp A, Voelkel S, Rupprecht L, Mueller T, Hilker M, Graf BM, Schmid C. Extracorporeal membrane oxygenation in severe trauma patients with bleeding shock. Resuscitation. 2010;81:804–9.
Fanelli V, Mascia L, Puntorieri V, Assenzio B, Elia V, Fornaro G, Martin EL, Bosco M, Delsedime L, Fiore T, Grasso S, Ranieri VM. Pulmonary atelectasis during low stretch ventilation: “open lung” versus “lung rest” strategy. Crit Care Med. 2009;37:1046–53.
Schmidt F, Sasse M, Boehne M, Mueller C, Bertram H, Kuehn C, Warnecke G, Ono M, Seidemann K, Jack T, Koeditz H. Concept of “awake venovenous extracorporeal membrane oxygenation” in pediatric patients awaiting lung transplantation. Pediatr Transplant. 2013;17:224–30.
Mohite PN, Sabashnikov A, Reed A, Saez DG, Patil NP, Popov AF, DeRobertis F, Bahrami T, Amrani M, Carby M, Kaul S, Simon AR. Extracorporeal life support in “awake” patients as a bridge to lung transplant. Thorac Cardiovasc Surg. 2015;63:699–705.
Borges JB, Porra L, Pellegrini M, Tannoia A, Derosa S, Larsson A, Bayat S, Perchiazzi G, Hedenstierna G. Zero expiratory pressure and low oxygen concentration promote heterogeneity of regional ventilation and lung densities. Acta Anaesthesiol Scand. 2016;60:958–68.
Guo L, Wang W, Zhao N, Guo L, Chi C, Hou W, Wu A, Tong H, Wang Y, Wang C, Li E. Mechanical ventilation strategies for intensive care unit patients without acute lung injury or acute respiratory distress syndrome: a systematic review and network meta-analysis. Crit Care. 2016;20:226.
Natalini G, Tuzzo D, Rosano A, Testa M, Grazioli M, Pennestri V, Amodeo G, Berruto F, Fiorillo M, Peratoner A, Tinnirello A, Filippini M, Marsilia PF, Minelli C, Bernardini A. Effect of external peep in patients under controlled mechanical ventilation with an auto-peep of 5 cmH2O or higher. Ann Intensive Care. 2016;6:53.
Nieszkowska A, Lu Q, Vieira S, Elman M, Fetita C, Rouby JJ. Incidence and regional distribution of lung overinflation during mechanical ventilation with positive end-expiratory pressure. Crit Care Med. 2004;32:1496–503.
Hong CM, Xu DZ, Lu Q, Cheng Y, Pisarenko V, Doucet D, Brown M, Aisner S, Zhang C, Deitch EA, Delphin E. Low tidal volume and high positive end-expiratory pressure mechanical ventilation results in increased inflammation and ventilator-associated lung injury in normal lungs. Anesth Analg. 2010;110:1652–60.
Dasenbrook EC, Needham DM, Brower RG, Fan E. Higher peep in patients with acute lung injury: a systematic review and meta-analysis. Respir Care. 2011;56:568–75.
Gothner M, Buchwald D, Schlebes A, Strauch JT, Schildhauer TA, Swol J. Use of extracorporeal membrane oxygenation in combination with high-frequency oscillatory ventilation in post-traumatic ARDS. Acta Anaesthesiol Scand. 2013;57:391–4.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors have no conflicts of interest to disclose.
Rights and permissions
About this article
Cite this article
Swol, J., Fülling, Y., Ull, C. et al. 48 h cessation of mechanical ventilation during venovenous extracorporeal membrane oxygenation in severe trauma: a case report. J Artif Organs 20, 280–284 (2017). https://doi.org/10.1007/s10047-017-0949-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10047-017-0949-6