Abstract
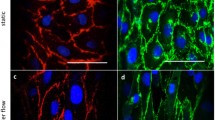
Human endothelial cells are used in experimental models for studying in vitro pathophysiological mechanisms of different diseases. We developed an original bioreactor, which can simulate human blood vessel, with capillary polysulfone membranes covered with the human umbilical vein endothelial cells (HUVECs) and we characterized its properties. The elaborated cell seeding and culturing procedures ensured formation of a confluent cell monolayer on the inside surface of capillaries within 24 h of culturing under the shear stress of 6.6 dyn/cm2. The optimal density of cells to be seeded was 60,000 cells/cm2. Labeling HUVECs with carboxyfluorescein succinimidyl ester (CFSE) did not influence cells’ metabolism. Flow cytometry-based analysis of HUVECs stained with CFSE demonstrated that in a presence of the shear stress cells’ proliferation was much inhibited (after 72 h proliferation index was equal to 1.9 and 6.2 for cultures with and without shear stress, respectively) and the monolayer was formed mainly due to migration and spreading of cells that were physiologically elongated in a direction of the flow. Monitoring of cells’ metabolism showed that HUVECs cultured in a presence of the shear stress preferred anaerobic metabolism and they consumed 1.5 times more glucose and produced 2.3 times more lactate than the cells cultured under static conditions. Daily von Willebrand factor production by HUVECs was near 2 times higher in a presence of the shear stress. The developed model can be used for at least 3 days in target studies under conditions mimicking the in vivo state more closely than the static HUVEC cultures.




Similar content being viewed by others
References
Mitchell JA, Ali F, Bailey L, Moreno L, Harrington LS. Role of nitric oxide and prostacyclin as vasoactive hormones released by the endothelium. Exp Physiol. 2008;93:141–7.
Sumpio BE, Riley JT, Dardik A. Cells in focus: endothelial cell. Int J Biochem Cell Biol. 2002;34:1508–12.
Watkins NV, Caro CG, Wang W. Parallel-plate flow chamber for studies of 3D flow-endothelium interaction. Biorheology. 2002;39:337–42.
Brown A, Burke G, Meenan BJ. Modeling of shear stress experienced by endothelial cells cultured on microstructured polymer substrates in a parallel plate flow chamber. Biotechnol Bioeng. 2011;108:1148–58.
Janke D, Jankowski J, Rüth M, Buschmann I, Lemke HD, Jacobi D, et al. The “artificial artery” as in vitro perfusion model. PLoS One. 2013;8:e57227.
Inoguchia H, Tanaka T, Maehara Y, Matsud T. The effect of gradually graded shear stress on the morphological integrity of a HUVEC-seeded compliant small-diameter vascular graft. Biomaterials. 2007;28:486–95.
Topper JN, Gimbrone MA Jr. Blood flow and vascular gene expression: fluid shear stress as a modulator of endothelial phenotype. Mol Med Today. 1999;5:40–6.
Chien S. Mechanotransduction and endothelial cell homeostasis: the wisdom of the cell. Am J Physiol Heart Circ Physiol. 2007;292:1209–24.
Ciechanowska A, Ladyzynski P, Hoser G, Sabalinska S, Kawiak J, Foltynski P, et al. Transmembrane pressure as an indicator of a density of endothelial cells cultured inside capillaries of a membrane bioreactor under dynamic conditions. IFMBE Proc. 2015;45:545–8.
Ciechanowska A, Schwanzer-Pfeiffer D, Rossmanith E, Sabalinska S, Wojciechowski C, Hartmann J, et al. Artificial vessel as a basis for disease related cell culture model. IFMBE Proc 2004;6.
Zolnierowicz J, Ambrozek-Latecka M, Kawiak J, Wasilewska D, Hoser G. Monitoring cell proliferation in vitro with different cellular fluorescent dyes. Folia Histochem Cytobiol. 2013;51:193–200.
Li YS, Haga JH, Chien S. Molecular basis of the effects of shear stress on vascular endothelial cells. J Biomech. 2005;38:1949–71.
Stoltz JF. Regenerative medicine and cell therapy. Influence of mechanical forces on cells and tissues. Amsterdam: IOS Press; 2012. p. 119–22.
Galbusera M, Zoja C, Donadelli R, Paris S, Morigi M, Benigni A, Figliuzzi M, Remuzzi G, Remuzzi A. Fluid shear stress modulates von Willebrand factor release from human vascular endothelium. Blood. 1997;90:1558–64.
Morigi M, Zoja C, Figliuzzi M, Foppolo M, Micheletti G, Bontempelli M, Saronni M, Remuzzi G, Remuzzi A. Fluid shear stress modulates surface expression of adhesion molecules by endothelial cells. Blood. 1995;85:1696–703.
Tsuboi H, Ando J, Korenaga R, Takada Y, Kamiya A. Flow stimulates ICAM-1 expression time and shear stress dependently in cultured human endothelial cells. Biochem Biophys Res Commun. 1995;206:988–96.
Witkowska AM, Borawska MH. Soluble intercellular adhesion molecule-1 (sICAM-1): an overview. Eur Cytokine Netw. 2004;15:91–8.
Wijerante SS, Li J, Yeh HC, Nolasco L, Zhou Z, Bergeron A, Frey EW, et al. Single-molecule force measurements of the polymerizing dimeric subunit of von Willebrand factor. Phys Rev E Stat Nonlinear Soft Matter Phys. 2016;93:012410.
Verdegem D, Moens S, Stapor P, Carmeliet P. Endothelial cell metabolism: parallels and divergences with cancer cell metabolism. Cancer Metab. 2014;15:2–19.
Harjes U, Bensaad K, Harris AL. Endothelial cell metabolism and implications for cancer therapy. Br J Cancer. 2012;107:1207–12.
Parra-Bonilla G, Alvarez DF, Al-Mehdi AB, Alexeyev M, Stevens T. Critical role for lactate dehydrogenase A in aerobic glycolysis that sustains pulmonary microvascular endothelial cell proliferation. Am J Physiol Lung Cell Mol Physiol. 2010;299:513–22.
Beckert S, Farrahi F, Aslam RS, Scheuenstuhl H, Königsrainer A, Hussain MZ, et al. Lactate stimulates endothelial cell migration. Wound Repair Regen. 2006;14:321–4.
Végran F, Boidot R, Michiels C, Sonveaux P, Feron O. Lactate influx through the endothelial cell monocarboxylate transporter MCT1 supports NF-κB/IL-8 pathway that drives tumor angiogenesis. Cancer Res. 2011;71:2550–60.
Hsu PP, Li S, Li YS, Usami S, Ratcliffe A, Wang X, et al. Effects of flow patterns on endothelial cell migration into a zone of mechanical denudation. Biochem Biophys Res Commun. 2001;285:751–9.
Hu YL, Li S, Miao H, Tsou TC, del Pozo MA, Chien S. Roles of microtubule dynamics and small GTPase Rac in endothelial cell migration and lamellipodium formation under flow. J Vasc Res. 2002;39:465–76.
Tkachenko E, Gutierrez E, Ginsberg MH, Groisman A. An easy to assemble microfluidic perfusion device with a magnetic clamp. Lab Chip. 2009;9:1085–95.
Acknowledgments
The studies presented in this paper were financed by research Grant No. N N518 290240 from the National Science Centre Poland.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors of this manuscript have competing interests.
Rights and permissions
About this article
Cite this article
Ciechanowska, A., Ladyzynski, P., Hoser, G. et al. Human endothelial cells hollow fiber membrane bioreactor as a model of the blood vessel for in vitro studies. J Artif Organs 19, 270–277 (2016). https://doi.org/10.1007/s10047-016-0902-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10047-016-0902-0