Abstract
Fulminant myocarditis is a rare but fatal serious disease that may cause prolonged native cardiac dysfunction with multiorgan failure despite temporary mechanical circulatory support with percutaneous venoatrial extracorporeal membrane oxygenation (VA-ECMO) or intraaortic balloon pumping (IABP). A 26-year-old man with fulminant myocarditis developed life-threatening multiorgan failure after 8 days support by VA-ECMO and IABP. He was transferred to our institution with prolonged cardiac dysfunction on hospital day 8; massive pulmonary edema developed into severe pulmonary dysfunction. Immediately after admission, VA-ECMO and IABP were switched to a paracorporeal pneumatic left ventricular assist device (LVAD) and right centrifugal ventricular assist device with an ECMO circuit shunting from the right ventricle to the pulmonary artery (RVAD-ECMO). After intensive care focusing on respiratory dysfunction, ECMO was successfully weaned, and the right ventricular assist device was switched to a durable paracorporeal pneumatic right ventricular assist device. The paracorporeal bi-ventricular assist devices were finally replaced with an implantable non-pulsatile LVAD on hospital day 181. Currently, 1 year after discharge, the patient is at home awaiting heart transplantation. Combined LVAD and RVAD-ECMO appear to be useful for resolving severe pulmonary edema due to unnecessarily long VA-ECMO support as well as kidney or liver dysfunction caused by circulatory collapse.
Similar content being viewed by others
Introduction
Fulminant myocarditis is a serious, sudden-onset disease that results in severe hemodynamic instability due to cardiac dysfunction and necessitates temporal mechanical circulatory support (MCS). Patients who survive the acute-phase crisis of fulminant myocarditis may have a favorable long-term outcome [1–3]. Given the complications of VA-ECMO, including multiorgan failure (MOF), it is necessary to switch this treatment to a ventricular assist device (VAD) at an appropriate time if native cardiac function is unlikely to recover. However, there is no established indication for VAD conversion and no optimal therapeutic strategy for fulminant myocarditis complicated by MOF under VA-ECMO support.
We present a case of fulminant myocarditis with MOF including severe pulmonary edema under prolonged VA-ECMO support that was successfully rescued with bi-ventricular assist devices (bi-VADs).
Case report
A previously healthy 26-year-old man was admitted to a local hospital and was diagnosed as acute myocarditis. After admission, the patient’s condition deteriorated drastically and VA-ECMO and IABP were initiated. Serum levels of creatine kinase and its MB isoenzyme peaked at 15,445 and 500 IU/L, respectively, on hospital day 3.
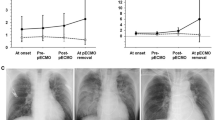
Cardiac function did not recover, and the patient gradually developed MOF, including severe pulmonary edema (Fig. 1a, b). He was transferred to our institute on hospital day 8. TTE showed severe bilateral ventricular dysfunction and LV dilatation [LV diastolic and systolic dimensions (LVDd and LVDs) of 61 and 59 mm, respectively; LV ejection fraction (LVEF), 10 %]. The aortic valve remained closed even in systole on VA-ECMO, and the total bilirubin level was elevated to 6.2 mg/dL. Renal function was preserved, with blood urea nitrogen and creatinine levels of 20 and 0.83 mg/dL, respectively. Although the patient required continuous hemodialytic assistance, he could pass urine without assistance. The partial oxygen pressure in the right radial artery was 336 mmHg under ventilator support with 60 % oxygen. Notably, pulmonary compliance was completely impaired because of massive pulmonary edema (tidal volume <50 mL).
Chest X-ray and computed tomography images showing severe pulmonary edema immediately before paracorporeal pneumatic left ventricular assist device (LVAD) and right centrifugal ventricular assist device with extracorporeal membrane oxygenation (RVAD-ECMO) (a, b); improved pulmonary edema before RVAD removal and conversion to an implantable LVAD (c)
As soon as the patient was transferred to our institution, VA-ECMO and IABP were switched to a Nipro-TOYOBO paracorporeal pneumatic LVAD (Nipro; Osaka, Japan) and right centrifugal VAD with ECMO (RVAD-ECMO). RVAD-ECMO was established using an extracorporeal life support system (Endumo® 6000, Heiwa Bussan; Tokyo, Japan), which consists of a ROTAFLOW® centrifugal pump (Maquet; Rastatt, Germany) and a circuit (T-NCVC® coating; National Cardiovascular Center; Osaka, Japan, and Toyobo; Osaka, Japan) as well as an oxygen membrane (BIOCUBE® 6000, Nipro; Osaka, Japan). The outflow and inflow cannula for RVAD were placed from the right ventricle (RV) to the pulmonary artery anticipating long-term support and conversion to paracorporeal RVAD (Fig. 2a). Lymphocytic myocarditis was definitively diagnosed by histological evaluation of samples from both the LV and RV apex, and the exceedingly high cardiac enzyme levels suggested that native cardiac function was not recoverable.
Multidisciplinary approach for multi-organ failure (a). Schema of paracorporeal pneumatic LVAD and RVAD-ECMO (b). Biphasic cuirass ventilation, a respiratory therapy for pulmonary dysfunction due to severe pulmonary edema, can provide appropriate artificial ventilation by means of external high-frequency oscillation via a cuirass tightly fitted around the patient’s chest
The patient’s hemodynamic condition was stable with RVAD-ECMO at a blood flow rate of 4.5 L/min. We predicted the flow rate of the LVAD by attaching an ultrasonic flowmeter to the inflow cannula of the LVAD and adjusted the VAD settings by performing echocardiography. Airway pressure release ventilation, biphasic cuirass ventilation (Fig. 2b), postural drainage, and transbronchial aspiration with a fiber bronchoscope were performed to treat acute respiratory distress syndrome due to severe pulmonary congestion. Oxygenation improved gradually after 12 days of intensive care (Fig. 1c). Nonetheless, RVAD-ECMO was switched to durable Nipro-TOYOBO paracorporeal pneumatic RVAD (Nipro) on hospital day 20 to treat the persistent RV failure. The patient was successfully weaned from mechanical ventilation on hospital day 52. The LVAD parameters on hospital day 84 were as follows: LVAD internal rate, 80 bpm; percent systole, 30 %; pressure, 200 mmHg; and vacuum, −45 mmHg, to maintain systemic circulation. By monitoring LVAD pump filling and echocardiographic findings, RVAD support was gradually weaned at an internal rate of up to 60 bpm, percent systole of 30 %, pressure of 100 mmHg, and vacuum of −35 mmHg, taking into account the risk of thrombus formation in the pump.
On hospital day 172, we performed the RVAD-off test using RHC and TTE. The patient was intravenously administered 3000 U heparin, and the RVAD internal rate was reduced by 20 bpm every 5 min under LVAD support with an LVAD internal rate of 80 bpm. Finally, RVAD was stopped for more than 5 min, at which time, the CI reduced from 3.45 to 2.51 L min−1 m−2 but was maintained, the mean pulmonary artery pressure (PAP) decreased from 26 to 21 mmHg, and the right atrial pressure increased slightly from 13 to 15 mmHg. Further, the systolic blood pressure and LVDd were almost unchanged compared to their values at an RVAD internal rate of 60 bpm. Moreover, pulmonary vascular resistance was as low as 1.46 wood units. On the basis of these findings, we concluded that right heart failure did exist, but we thought that this could be managed by conversion to implantable continuous-flow LVAD (CF-LVAD) and volume reduction, medical therapy such as nitric oxide or dobutamine/PDE3 inhibitor infusion, and cardiac rehabilitation. The survival rate with bi-VAD support has remained inferior to that with isolated LVAD [4], and the anticipated waiting period for heart transplantation is now over 3 years; so we decided to wean the patient off the RVAD.
The patient’s native LV function did not recover at 3 months post-myocarditis onset (LVDd, 57 mm; LVDs, 52 mm; LVEF, 15 % under LVAD support). RV endomyocardial biopsy examination showed that the inflammation had resolved, and widespread replacement fibrosis was observed. Therefore, the patient was registered as a candidate for heart transplantation on hospital day 179, after 3 months of multiple antibiotic treatments for a concomitant severe right lung abscess.
On hospital day 181, the bi-VAD was replaced with the HeartMate II® (Thoratec Corporation; Pleasanton, CA, USA) implantable CF-LVAD. RHC data at 51 days after RVAD explantation and under Heart Mate II® support with a pump speed of 8600 rpm were mean blood pressure of 73 mmHg, mean PCWP of 8 mmHg, mean PAP of 16 mmHg, mean RAP of 9 mmHg, and CO/CI of 3.79 L min−1/2.37 L min−1 m−2. CO was low but maintained despite reduced LV preload.
The patient was finally discharged 2 months after the operation, and is currently awaiting heart transplantation while clinically stable under 8600 rpm support of HeartMate II®, 1 year after discharge.
Discussion
VA-ECMO simultaneously facilitates oxygenation and bilateral ventricular support, compensating for approximately 70 % of blood flow regardless of the patient’s cardiac function. However, the LV is not vented directly, and retrograde ECMO flow increases the LV afterload and end-diastolic pressure (LVEDP), leading to pulmonary congestion if managed inappropriately. Additionally, increased LV activity impairs the myocardial oxygen supply/demand balance, resulting in further myocardial damage. Concomitant IABP usually reduces LV afterload and increases coronary blood flow, but is insufficient for prolonged and high-flow VA-ECMO support. On the other hand, LVADs supply antegrade flow, which moves blood from the LV to the ascending aorta, thus yielding physiological hemodynamics, and the preserved RV function supports the required blood flow.
When managing VA-ECMO in patients with severe cardiac dysfunction, cardiac functional recovery should be evaluated under the minimum ECMO flow possible to allow the aortic valve to open. However, this is very difficult even with catecholamine infusion, when native cardiac function is severely deteriorated. If temporary MCS weaning is deemed difficult, rapid conversion to VAD is necessary before irreversible MOF develops.
Previous studies reported that VA-ECMO-associated MOF including liver and kidney dysfunction determines the outcome of fulminant myocarditis and is an indicator for the VAD conversion timing [5, 6]. As described above, pulmonary edema is another serious complication of VA-ECMO, and respiratory failure can become irreversible, thereby increasing the risk of a progressive respiratory tract infection. Pagani et al. also reported that pulmonary compliance was an important determinant of the outcome; ECMO patients with consistently poor pulmonary compliance (<25 cm3/cm H2O) had poor ECMO and LVAD outcomes [7].
Fulminant myocarditis usually affects both the LV and RV, but under VA-ECMO support, it is very difficult to estimate RV function precisely. In the present case of severe pulmonary dysfunction and subsequent pulmonary vascular resistance elevation, we actively implanted an RVAD-ECMO to simultaneously provide RV support and oxygenation in combination with a paracorporeal LVAD in the acute phase. We decided on this treatment because planned bi-VAD insertion results in a higher possibility of survival than delayed conversion of LVAD to bi-VAD [8]. Further, once the cardiomyocytic inflammation improves or pulmonary dysfunction recovers, this RVAD can be removed more easily than a paracorporeal RVAD. Although the timing of VAD conversion was severely delayed in our patient, MOF was treated successfully, and the patient survived because of LVAD and RVAD-ECMO.
In this case, RVAD-ECMO was switched to Nipro-TOYOBO RVAD on hospital day 20 to treat persistent RV failure that managed for more than 5 months. Management of Nipro-TOYOBO bi-VAD is difficult from the point of view of whole-body monitoring and adjustment of settings for two VADs. To adjust bi-VAD settings, we performed daily echocardiography after implantation, supplementarily checked central venous pressure and flow rate data obtained from an ultrasonic flowmeter attached to the inflow cannula.
In conclusion, combined LVAD and RVAD-ECMO seems useful for resolving the severe pulmonary edema resulting from VA-ECMO support as well as kidney or liver dysfunction caused by circulatory collapse. Evaluation of major organ functions and understanding the advantages and disadvantages of VA-ECMO assistance are essential to time VAD conversion correctly.
References
Maejima Y, Yasu T, Kubo N, Kawahito K, Omura N, Katsuki T, Tsukamoto Y, Sugawara Y, Hashimoto S, Tsuruya Y, Hirahara T, Takagi Y, Kobayashi N, Funayama H, Ikeda N, Ishida T, Fujii M, Ino T, Saito M. Long-term prognosis of fulminant myocarditis rescued by percutaneous cardiopulmonary support device. Circ J. 2004;68:829–33.
Cheng R, Hachamovitch R, Kittleson M, Patel J, Arabia F, Moriguchi J, Esmailian F, Azarbal B. Clinical outcomes in fulminant myocarditis requiring extracorporeal membrane oxygenation; a weighted meta-analysis of 170 patients. J Cardiac Fail. 2014;20:400–6.
Aoyama N, Izumi T, Hiramori K, Isobe M, Kawana M, Hiroe M, Hishida H, Kitaura M, Imaizumi T, Japanese Investigators of Fulminant Myocarditis. National survey of fulminant myocarditis in Japan: therapeutic guidelines and long-term prognosis of using percutaneous cardiopulmonary support for fulminant myocarditis (Special report from a scientific committee). Circ J. 2002;66:133–44.
Kirklin JK, Naftel DC, Pagani FD, Kormos RL, Stevenson LW, Blume ED, Myers SL, Miller MA, Baldwin JT, Young JB. Seventh INTERMACS annual report: 15000 patients and counting. J Heart Lung Transplant. 2015;24:1495–504.
Peek GJ, Killer HM, Sosnowski MA, Firmin RK. Modular extracorporeal life support for multiorgan failure patients. Liver. 2002;22:69–71.
Reinhartz O, Farrar DJ, Hershon JH, Avery GJ Jr, Haeusslein EA, Hill JD. Importance of preoperative liver function as a predictor of survival in patients supported with Thoratec ventricular assist device as a bridge to transplantation. J Thorac Cardiovasc Surg. 1998;116:633–40.
Pagani FD, Lynch W, Swaniker F, Dyke DB, Bartlett R, Koelling T, Moscucci M, Deeb GM, Bolling S, Monaghan H, Aaronson KD. Extracorporeal life support to left ventricular assist device bridge to heart transplant: a strategy to optimize survival and resource utilization. Circulation. 1999;100:22306–10.
Fitzpatrick JR 3rd, Frederick JR, Hiesinger W, Hsu VM, McCormick RC, Kozin ED, Laporte CM, O’Hara ML, Howell E, Dougherty D, Cohen JE, Southerland KW, Howard JL, Paulson EC, Acker MA, Morris RJ, Woo YJ. Early, planned institution of biventricular mechanical circulatory support results in improved outcomes compared to delayed conversion of LVAD to BiVAD. J Thorac Cardiovasc Surg. 2009;137:971–7.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Nakajima, S., Seguchi, O., Fujita, T. et al. Successful treatment of near-fatal fulminant myocarditis using bi-ventricular assist device support. J Artif Organs 19, 293–296 (2016). https://doi.org/10.1007/s10047-016-0899-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10047-016-0899-4