Abstract
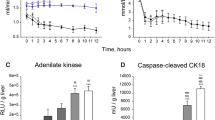
The multiorgan ex vivo perfused liver–kidney model allows studying the hepatic pathophysiology and purifying waste products. We tested if the addition of the kidney first followed by the liver (KL circuit) produces better results compared to the classic liver-first approach (LK). Intact livers and kidneys were obtained post mortem from ten female domestic white pigs, five experiments were conducted with the KL circuit and five with the LK. Bile, urine production, arterial blood gases, glucose, renal and liver tests were collected hourly during the perfusions. The KL circuit had values more close to physiological ranges, more stable over time and showed less variability compared to the LK circuit for urine production, glucose, PH, anion gap, lactate, urea, sodium, potassium and Alanine Transaminase (ANOVA test for repeated measures p < 0.05). The KL circuit produced a more physiological and reliable biochemical milieu.




Similar content being viewed by others
References
Gravante G, Ong SL, McGregor A, Sorge R, Metcalfe MS, Lloyd DM, Dennison AR. Histological changes during extracorporeal perfusions of the porcine liver: implications for temporary support during acute liver failures. J Artif Organs. 2012;16:218–28.
Alzaraa A, Al-Leswas D, Chung WY, Gravante G, Bruno M, West K, Dennison A, Lloyd D. Contrast-enhanced ultrasound detects perfusion defects in an ex vivo porcine liver model: a useful tool for the study of hepatic reperfusion. J Artif Organs [Epub ahead of print], 2013.
Gravante G, Ong SL, Metcalfe MS, Sorge R, Sconocchia G, Orlando G, Lloyd DM, Dennison AR. Cytokine response to ischemia/reperfusion injury in an ex vivo perfused porcine liver model. Transplant Proc. 2009;41:1107–12.
Vogel T, Brockmann JG, Friend PJ. Ex-vivo normothermic liver perfusion: an update. Curr Opin Organ Transplant. 2010;15:167–72.
Bellomo R, Suzuki S, Marino B, Starkey GK, Chambers B, Fink MA, Wang BZ, Houston S, Eastwood G, Calzavacca P, Glassford N, Skene A, Jones DA, Jones R. Normothermic extracorporeal perfusion of isolated porcine liver after warm ischaemia: a preliminary report. Crit Care Resusc. 2012;14:173–6.
Jamieson RW, Zilvetti M, Roy D, Hughes D, Morovat A, Coussios CC, Friend PJ. Hepatic steatosis and normothermic perfusion-preliminary experiments in a porcine model. Transplantation. 2011;92:289–95.
Stadler M, Nuyens V, Seidel L, Albert A, Boogaerts JG. Effect of nutritional status on oxidative stress in an ex vivo perfused rat liver. Anesthesiology. 2005;103:978–86.
Mechet I, Lhuillier F, Blanchet MC, Pouyet M, Viale JP, Goudable J, Annat G, Scoazec JY, Boillot O, Liotard D, Merle E, Delafosse B. Liver function during extracorporeal whole liver perfusion in a pig model of acute ischemic liver failure. ASAIO J. 2004;50:503–11.
St Peter SD, Imber CJ, Friend PJ. Liver and kidney preservation by perfusion. Lancet. 2002;359:604–13.
Vogel T, Brockmann JG, Coussios C, Friend PJ. The role of normothermic extracorporeal perfusion in minimizing ischemia reperfusion injury. Transplant Rev Orlando Fla. 2012;26:156–62.
Gravante G, Ong SL, Metcalfe MS, Sorge R, Fox AJ, Lloyd DM, Maddern GJ, Dennison AR. Changes in acid-base balance during electrolytic ablation in an ex vivo perfused liver model. Am J Surg, 2010. [Epub ahead of print].
Gravante G, Ong SL, Metcalfe MS, Sorge R, Overton J, Lloyd DM, Maddern GJ, Dennison AR. Cytokine response of electrolytic ablation in an ex vivo perfused liver model. ANZ J Surg. 2010;80:537–41.
Dodd GD 3rd, Dodd NA, Lanctot AC, Glueck DA. Effect of variation of portal venous blood flow on radiofrequency and microwave ablations in a blood-perfused bovine liver model. Radiology. 2013;267:129–36.
Gravante G, Ong SL, Metcalfe MS, Sorge R, Bikhchandani J, Lloyd DM, Dennison AR. Effects of hypoxia due to isovolemic hemodilution on an ex vivo normothermic perfused liver model. J Surg Res. 2010;160:73–80.
Gravante G, Ong SL, Metcalfe M, Lloyd D, Dennison A. The porcine hepatic arterial supply, its variations and their influence on the extracorporeal perfusion of the liver. J Surg Res, 2009.
Schön MR, Kollmar O, Wolf S, Schrem H, Matthes M, Akkoc N, Schnoy NC, Neuhaus P. Liver transplantation after organ preservation with normothermic extracorporeal perfusion. Ann Surg. 2001;233:114–23.
Chung W, Gravante G, Al-Leswas D, Alzaraa A, Sorge R, Ong S, Pollard C, Lloyd D, Metcalfe M, Dennison A. The autologous normothermic ex vivo perfused porcine liver-kidney model: improving the circuit’s biochemical and acid-base environment. Am J Surg, 2012. (in press).
Chung WY, Gravante G, Al-Leswas D, Alzaraa A, Sorge R, Ong SL, Pollard C, Lloyd DM, Metcalfe, MS, Dennison AR. Addition of a kidney to the normothermic ex vivo perfused porcine liver model does not increase cytokine response. J Artif Organs, 2012.
Chung WY, Gravante G, Al-Leswas D, Arshad A, Sorge R, Watson CC, Pollard C, Metcalfe MS, Dennison AR. The development of a multiorgan ex vivo perfused model: results with the porcine liver-kidney circuit over 24 hours. Artif Organs. 2013;37:457–66.
Behrends M, Walz MK, Kribben A, Neumann T, Helmchen U, Philipp T, Schulz R, Heusch G. No protection of the porcine kidney by ischaemic preconditioning. Exp Physiol. 2000;85:819–27.
Eckhauser F, Knol JA, Porter-Fink V, Lockery D, Edgcomb L, Strodel WE, Webb D, Simmons J. Ex vivo normothermic hemoperfusion of the canine pancreas: applications and limitations of a modified experimental preparation. J Surg Res. 1981;31:22–37.
Acknowledgments
We would like to thank Sarah Hosgood from the Department of Transplantation, University Hospitals of Leicester, for connecting the porcine kidney in this model and providing expert advice during the experiments setup.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chung, W.Y., Gravante, G., Eltweri, A. et al. The “kidney–liver” multiorgan ex vivo perfused model improves the circuit’s biochemical milieu during perfusion compared to the “liver–kidney” counterpart. J Artif Organs 18, 151–161 (2015). https://doi.org/10.1007/s10047-014-0813-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10047-014-0813-x