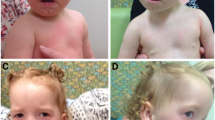
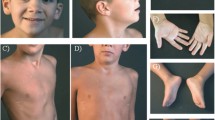
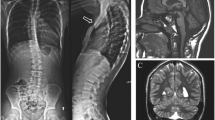
Abstract
Two cases of newborns with deletion 13q syndrome were identified and studied using electron microscopy and histologic, immunohistochemical, and special stained sections. We reviewed the published literature on genes that are haploinsufficient in the deletion 13q syndrome. The complexity of the deletion 13q syndrome phenotype is due at least in part to the haploinsufficiency of dosage-sensitive genes. Future studies need to be performed to identify their precise role in the cellular function and the development of the deletion 13q syndrome phenotype.







Similar content being viewed by others
References
Brown S, Russo J, Chitayat D, Warburton D. The 13q− syndrome: the molecular definition of a critical region in band 13q32. Am J Hum Genet 1995;57:857–866
Allderdice PW, Davis JG, Miller OJ, et al. The 13q-deletion syndrome. Am J Hum Genet 1969;21:499–512
Grace E, Drennan J, Colver D, Gordon RR. The 13q− deletion syndrome. J Med Genet 1971;8:351–357
Chemke J, Fishel E, Zalish M, Sagiv M. Multiple skeletal anomalies in the “13q−” syndrome. Eur J Pediatr 1978;128:27–31
Carnevale A, Frias S, Alcantar R. Interstitial deletion of long arm of chromosome 13. Ann Genet 1984;27:49–52
Brown SA, Warburton D, Brown LY, et al. Holoprosencephaly due to mutations in ZIC2, a homologue of Drosophila odd-paired. Nat Genet 1998;20:180–183
Nagai T, Aruga J, Takada S, et al. The expression of the mouse Zic1, Zic2, and Zic3 gene suggests an essential role for Zic genes in body pattern formation. Dev Biol 1997;182:299–313
Brown LY, Odent S, David V, et al. Holoprosencephaly due to mutations in ZIC2: alanine tract expansion mutations may be caused by parental somatic recombination. Hum Mol Genet 2001;10:791–796
Dalski A, Atici J, Kreuz FR, et al. Mutation analysis in the fibroblast growth factor 14 gene: frameshift mutation and polymorphisms in patients with inherited ataxias. Eur J Hum Genet 2005;13:118–120
Smallwood PM, Munoz-Sanjuan I, Tong P, et al. Fibroblast growth factor (FGF) homologous factors: new members of the FGF family implicated in nervous system development. Proc Natl Acad Sci 1996;93:9850–9857
Wang Q, Bardgett ME, Wong M, et al. Ataxia and paroxysmal dyskinesia in mice lacking axonally transported FGF14. Neuron 2002;35:25–38
van Swieten JC, Brusse E, de Graaf BM, et al. A mutation in the fibroblast growth factor 14 gene is associated with autosomal dominant cerebellar ataxia. Am J Hum Genet 2003;72:191–199
Malas S, Duthie S, Deloukas P, Episkopou V. The isolation and high-resolution chromosomal mapping of human SOX14 and SOX21; two members of the SOX gene family related to SOX1, SOX2, and SOX3. Mamm Genome 1999;10:934–937
Bylund M, Andersson E, Novitch BG, Muhr J. Vertebrate neurogenesis is counteracted by Sox1-3 activity. Nat Neurosci 2003;6:1162–1168
Kennedy JF, Freeman MG, Benirschke K. Ovarian dysgenesis and chromosomal abnormalities. Obstet Gynecol 1977;50:13–20
Cunniff C, Jones KL, Benirschke K. Ovarian dysgenesis in individuals with chromosomal abnormalities. Hum Genet 1991;86:552–556
Burks DJ, Font de Mora J, Schubert M, et al. IRS-2 pathways integrate female reproduction and energy homeostasis. Nature 2000;407:377–382
Tobe K, Suzuki R, Aoyama M, et al. Increased expression of the sterol regulatory element-binding protein-1 gene in insulin receptor substrate-2 −/− mouse liver. J Biol Chem 2001;276:38337–38340
Withers DJ, Gutierrez JS, Towery H, et al. Disruption of IRS-2 causes type 2 diabetes in mice. Nature 1998;391:900–902
Hennige AM, Burks DJ, Ozcan U, et al. Upregulation of insulin receptor substrate-2 in pancreatic beta-cells prevents diabetes. J. Clin. Invest 2003;112:1521–1532
Mammarella S, Romano F, Di Valerio A, et al. Interaction between the G1057D variant of IRS-2 and overweight in the pathogenesis of type 2 diabetes. Hum Mol Genet 2000;9:2517–2521
Poschl E, Pollner R, Kuhn K. The genes for the alpha-1(IV) and alpha-2(IV) chains of human basement membrane collagen type IV are arranged head-to-head and separated by a bidirectional promoter of unique structure. EMBO J 1988;7:2687–2695
Soininen R, Huotari M, Hostikka SL, et al. The structural genes for alpha-1 and alpha-2 chains of human type IV collagen are divergently encoded on opposite DNA strands and have an overlapping promoter region. J Biol Chem 1988;263:17217–17220
Tanaka N, Tajima S, Ishibashi A, et al. Expression of the alpha1-alpha6 collagen IV chains in the dermoepidermal junction during human foetal skin development: temporal and spatial expression of the alpha4 collagen IV chain in an early stage of development. Br J Dermatol 1998;139:371–374
Acknowledgments
We thank Marilyn Jones, MD, of Children’s Hospital in San Diego for reviewing the manuscript and Henry Powell, MD, and Larry Hanson, MD, from the Department of Pathology, University of California of San Diego, University Medical Center for direct contribution to this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kasyan, A.G., Benirschke, K. Genetic Haploinsufficiency as a Phenotypic Determinant of a Deletion 13q Syndrome. Pediatr Dev Pathol 8, 658–665 (2005). https://doi.org/10.1007/s10024-005-0066-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10024-005-0066-z