Abstract
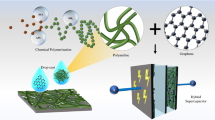
In the current work, the effect of aniline concentration on the polymerization process and supercapacitive behavior of graphene oxide/multiwalled carbon nanotubes/polyaniline (GMP) nanocomposites were studied. Based on the obtained results, GMP nanocomposite with 0.5 M aniline (GMP5) was selected as the optimum concentration in terms of high current density and high specific capacitance. Nafion-based ionic polymer-free metal composite (IPFMC) supercapacitor was fabricated for the GMP5 nanocomposite. Solid-state symmetric supercapacitor was made after spraying of GMP5 in. on both sides of Nafion membrane. The electrochemical properties were investigated by cyclic voltammetry (CV), galvanostatic charge–discharge (CD), and electrochemical impedance spectroscopy (EIS) techniques in 0.5 M Na2SO4.The specific capacitance of 383.25 F g−1 (326 mF cm−2) and 527.5 F g−1 (42 mF cm−2) was obtained for the GMP5 in solid-state supercapacitor and three-electrode cell at a scan rate of 10 mV s−1, respectively. The maximum energy and power densities of 53.64 and 1777.4 W kg−1 were obtained for the IPFMC-based supercapacitor.

Schematic of the solid-state supercapacitor based on the GMP5 nanocomposite














Similar content being viewed by others
References
Hosseini MG, Shahryari E (2016) Performance of polyaniline/manganese oxide-MWCNT nanocomposites as supercapacitors. Iran Chem Commun 3:67–77
Hosseini MG, Shahryari E, Najjar R, Ahadzadeh I (2015) Study of supercapacitive behavior of polyaniline/manganese oxide-carbon black nanocomposites based electrodes. Int J Nanosci Nanotechnol 11:147–157
Cheng L, Li H-q, Xia Y-y (2006) A hybrid nonaqueous electrochemical supercapacitor using nano-sized iron oxyhydroxide and activated carbon. J Solid State Electrochem 10(6):405–410
Parra-Elizondo V, Escobar-Morales B, Morales E, Pacheco-Catalán D (2016) Effect of carbonaceous support between graphite oxide and reduced graphene oxide with anchored Co3O4 microspheres as electrode-active materials in a solid-state electrochemical capacitor. J Solid State Electrochem 21(4):975–985
Wei W, Cui X, Chen W, Ivey DG (2011) Manganese oxide-based materials as electrochemical supercapacitor electrodes. Chem Soc Rev 40(3):1697–1721
Zhang Y, Si L, Zhou B, Zhao B, Zhu Y, Zhu L, Jiang X (2016) Synthesis of novel graphene oxide/pristine graphene/polyaniline ternary composites and application to supercapacitor. Chem Eng J 288:689–700
Lewandowski A, Olejniczak A, Galinski M, Stepniak I (2010) Performance of carbon–carbon supercapacitors based on organic, aqueous and ionic liquid electrolytes. J Power Sources 195(17):5814–5819
Xu D, Xu Q, Wang K, Chen J, Chen Z (2014) Fabrication of free-standing hierarchical carbon nanofiber/graphene oxide/polyaniline films for supercapacitors. Appl Mat Interfaces 6:200–209
Aboutalebi SH, Chidembo AT, Salari M, Konstantinov K, Wexler D, Liu HK, Dou SX (2011) Comparison of GO, GO/MWCNTs composite and MWCNTs as potential electrode materials for supercapacitors. Energy Environ Sci 4(5):1855
Xia X, Lei W, Hao Q, Wang W, Wang X (2013) One-step synthesis of CoMoO4/graphene composites with enhanced electrochemical properties for supercapacitors. Electrochim Acta 99:253–261
Khalid M, Tumelero MA, Pasa AA (2015) Asymmetric and symmetric solid-state supercapacitors based on 3D interconnected polyaniline-carbon nanotube framework. RSC Adv 5(76):62033–62039
Vermisoglou EC, Giannakopoulou T, Romanos GE, Boukos N, Giannouri M, Lei C, Lekakou C, Trapalis C (2015) Non-activated high surface area expanded graphite oxide for supercapacitors. Appl Surf Sci 358(Part A):110–121
Lazzari M, Soavi F, Mastragostino M (2009) Dynamic pulse power and energy of ionic-liquid-based supercapacitor for HEV application. J Electrochem Soc 156(8):A661–A666
Fan W, Miao Y-E, Zhang L, Huang Y, Liu T (2015) Porous graphene-carbon nanotube hybrid paper as a flexible nano-scaffold for polyaniline immobilization and application in all-solid-state supercapacitors. RSC Adv 5(39):31064–31073
Mousa MA, Khairy M, Shehab M (2016) Nanostructured ferrite/graphene/polyaniline using for supercapacitor to enhance the capacitive behavior. J Solid State Electrochem 21(4):995–1005
Snook GA, Kao P, Best AS (2011) Conducting-polymer-based supercapacitor devices and electrodes. J Power Sources 196(1):1–12
Wang R, Wu Q, Zhang X, Yang Z, Gao L, Ni J, Tsui OKC (2016) Flexible supercapacitors based on a polyaniline nanowire-infilled 10 nm-diameter carbon nanotube porous membrane by in situ electrochemical polymerization. J Mater Chem A 4(32):12602–12608
Kurig H, Vestli M, Tõnurist K, Jänes A, Lust E (2012) Influence of room temperature ionic liquid anion chemical composition and electrical charge delocalization on the supercapacitor properties. J Electrochem Soc 159(7):A944–A951
Khosrozadeh A, Xing M, Wang Q (2015) A high-capacitance solid-state supercapacitor based on free-standing film of polyaniline and carbon particles. Appl Energy 153:87–93
Shabani Shayeh J, Ehsani A, Ganjali MR, Norouzi P, Jaleh B (2015) Conductive polymer/reduced graphene oxide/Au nano particles as efficient composite materials in electrochemical supercapacitors. Appl Surf Sci 353:594–599
Zhou H, Chen H, Luo S, Lu G, Wei W, Kuang Y (2005) The effect of the polyaniline morphology on the performance of polyaniline supercapacitors. J Solid State Electrochem 9(8):574–580
Xie Y, Xia C, Du H, Wang W (2015) Enhanced electrochemical performance of polyaniline/carbon/titanium nitride nanowire array for flexible supercapacitor. J Power Sources 286:561–570
Ma B, Zhou X, Bao H, Li X, Wang G (2012) Hierarchical composites of sulfonated graphene-supported vertically aligned polyaniline nanorods for high-performance supercapacitors. J Power Sources 215:36–42
Hou Y, Cheng Y, Hobson T, Liu J (2010) Design and synthesis of hierarchical MnO2 nanospheres/carbon nanotubes/conducting polymer ternary composite for high performance electrochemical electrodes. Nano Lett 10(7):2727–2733
Zhi M, Xiang C, Li J, Li M, Wu N (2012) Nanostructured carbon–metal oxide composite electrodes for supercapacitors: a review. Nano 5:72–88
Hosseini MG, Shahryari E (2017) A novel high-performance supercapacitor based on chitosan/GO-MWCNT/PANI. J Colloid Interface Sci 496:371–381
Yoon S-B, Yoon E-H, Kim K-B (2011) Electrochemical properties of leucoemeraldine, emeraldine, and pernigraniline forms of polyaniline/multi-wall carbon nanotube nanocomposites for supercapacitor applications. J Power Sources 196(24):10791–10797
Lufrano F, Staiti P (2004) Conductivity and capacitance properties of a supercapacitor based on Nafion electrolyte in a nonaqueous system. Electrochem Solid-State Lett 7(11):A447–A450
Hosseini MG, Rasouli H, Shahryari E, Naji L (2017) Electrochemical behavior of a Nafion-membrane-based solid-state supercapacitor with a graphene oxide–multiwalled carbon nanotube–polypyrrole nanocomposite. J Appl Polym Sci:44926
Electroactive Polymer (EAP) (2004) Actuators as artificial muscles: reality, potential, and challenges, 2nd edition. SPIE PM136:1–816
Naji L, Chudek JA, Abel EW, Baker RT (2013) Electromechanical behaviour of Nafion-based soft actuators. J Mater Chem B 1(19):2502–2514
Hummers WS, Offman RE (1958) Preparation of graphitic oxide. J American Chem Soc 80(6):1339–1339
Hosseini MG, Shahryari E Synthesis, characterization and electrochemical study of graphene oxide-multi walled carbon nanotube-manganese oxide-polyaniline-electrode as supercapacitor. J Mater Sci & Technol 32:763–773
Tang Y, Chen C, Ye YS, Xue Z, Zhou X, Xie X (2014) The enhanced actuation response of an ionic polymer-metal composite actuator based on sulfonated polyphenylsulfone. Polym Chem 5(20):6097–6107
Naji L, Chudek JA, Baker RT (2008) Magnetic resonance imaging study of a soft actuator element during operation. Soft Matter 4(9):1879–1886
Yan Y, Cheng Q, Pavlinek V, Saha P, Li C (2012) Fabrication of polyaniline/mesoporous carbon/MnO2 ternary nanocomposites and their enhanced electrochemical performance for supercapacitors. Electrochim Acta 71:27–32
Mirmohseni A, Dorraji MSS, Hosseini MG (2012) Influence of metal oxide nanoparticles on pseudocapacitive behavior of wet-spun polyaniline-multiwall carbon nanotube fibers. Electrochim Acta 70:182–192
Chen C-M, Huang J-Q, Zhang Q, Gong W-Z, Yang Q-H, Wang M-Z, Yang Y-G (2012) Annealing a graphene oxide film to produce a free standing high conductive graphene film. Carbon 50(2):659–667
Golczak S, Kanciurzewska A, Fahlman M, Langer K, Langer JJ (2008) Comparative XPS surface study of polyaniline thin films. Solid State Ionics 179(39):2234–2239
Xie B, Wang Y, Lai W, Lin W, Lin Z, Zhang Z, Zou P, Xu Y, Zhou S, Yang C, Kang F, Wong C-P (2016) Laser-processed graphene based micro-supercapacitors for ultrathin, rollable, compact and designable energy storage components. Nano Energy 26:276–285
Yan W, Ayvazian T, Kim J, Liu Y, Donavan KC, Xing W, Yang Y, Hemminger JC, Penner RM (2011) Mesoporous manganese oxide nanowires for high-capacity, high-rate, hybrid electrical energy storage. ACS Nano 5(10):8275–8287
Xu J, Wang K, Zu S-Z, Han B-H, Wei Z (2010) Hierarchical nanocomposites of polyaniline nanowire arrays on graphene oxide sheets with synergistic effect for energy storage. ACS Nano 4:5019–5026
Conway BE (1999) Electrochemical supercapacitors scientific fundamentals and technological applications. Kluwer Academic Plenum Publisher, New york
Mi H, Zhou J, Cui Q, Zhao Z, Yu C, Wang X, Qiu J (2014) Chemically patterned polyaniline arrays located on pyrolytic graphene for supercapacitors. Carbon 80:799–807
Abdulhakeem B, Farshad B, Damilola M, Fatemeh T, Mopeli F, Julien D, Ncholu M (2014) Morphological characterization and impedance spectroscopy study of porous 3D carbons based on graphene foam-PVA/phenol-formaldehyde resin composite as an electrode material for supercapacitors. RSC Adv 4(73):39066–39072
Ren G, Pan X, Bayne S, Fan Z (2014) Kilohertz ultrafast electrochemical supercapacitors based on perpendicularly-oriented graphene grown inside of nickel foam. Carbon 71:94–101
Taberna P, Simon P, Fauvarque J-F (2003) Electrochemical characteristics and impedance spectroscopy studies of carbon-carbon supercapacitors. J Electrochem Soc 150(3):A292–A300
Yan J, Sun W, Wei T, Zhang Q, Fan Z, Wei F (2012) Fabrication and electrochemical performances of hierarchical porous Ni(OH)2 nanoflakes anchored on graphene sheets. J Mater Chem 22(23):11494–11502
Zhao X, Chen C, Huang Z, Jin L, Zhang J, Li Y, Zhang L, Zhang Q (2015) Rational design of polyaniline/MnO2/carbon cloth ternary hybrids as electrodes for supercapacitors. RSC Adv 5(81):66311–66317
Li Y, Zhao X, Xu Q, Zhang Q, Chen D (2011) Facile preparation and enhanced capacitance of the polyaniline/sodium alginate nanofiber network for supercapacitors. Langmuir 27(10):6458–6463
Yang X, Zhang F, Zhang L, Zhang T, Huang Y, Chen Y (2013) A high-performance graphene oxide-doped ion gel as gel polymer electrolyte for all-solid-state supercapacitor applications. Adv Funct Mater 23(26):3353–3360
Devarayan K, Lei D, Kim H-Y, Kim B-S (2015) Flexible transparent electrode based on PANi nanowire/nylon nanofiber reinforced cellulose acetate thin film as supercapacitor. Chem Eng J 273:603–609
Zhang L, Zhu P, Zhou F, Zeng W, Su H, Li G, Gao J, Sun R, Wong C-p (2016) Flexible asymmetrical solid-state supercapacitors based on laboratory filter paper. ACS Nano 10(1):1273–1282
Gao Z, Yang W, Wang J, Song N, Li X (2015) Flexible all-solid-state hierarchical NiCo2O4/porous graphene paper asymmetric supercapacitors with an exceptional combination of electrochemical properties. Nano Energy 13:306–317
Yan D, Li Y, Liu Y, Zhuo R, Geng B, Wu Z, Wang J, Ren P, Yan P (2015) Design and influence of mass ratio on supercapacitive properties of ternary electrode material reduced graphene oxide@MnO2@ poly(3,4-ethylenedioxythiophene)-poly(styrene sulfonate). Electrochim Acta 169:317–325
Wang D-W, Li F, Zhao J, Ren W, Chen Z-G, Tan J, Wu Z-S, Gentle I, Lu GQ, Cheng H-M (2009) Fabrication of graphene/polyaniline composite paper via in situ anodic electropolymerization for high-performance flexible electrode. ACS Nano 3(7):1745–1752
Lu X, Dou H, Yang S, Hao L, Zhang L, Shen L, Zhang F, Zhang X (2011) Fabrication and electrochemical capacitance of hierarchical graphene/polyaniline/carbon nanotube ternary composite film. Electrochim Acta 56(25):9224–9232
Yan Y, Cheng Q, Zhu Z, Pavlinek V, Saha P, Li C (2013) Controlled synthesis of hierarchical polyaniline nanowires/ordered bimodal mesoporous carbon nanocomposites with high surface area for supercapacitor electrodes. J Power Sources 240:544–550
Gui D, Liu C, Chen F, Liu J (2014) Preparation of polyaniline/graphene oxide nanocomposite for the application of supercapacitor. Appl Surf Sci 307:172–177
Acknowledgments
The authors would like to acknowledge the financial support of the Iranian National Committee of Nanotechnology in Ministry of Science, Research and Technology and the office of the Vice Chancellor in Charge of Research of University of Tabriz. The authors would like to thank O. Mermer and A. Farzaneh for the assistance in the measurement of XPS spectra.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hosseini, M.G., Shahryari, E. Fabrication of novel solid-state supercapacitor using a Nafion polymer membrane with graphene oxide/multiwalled carbon nanotube/polyaniline. J Solid State Electrochem 21, 2833–2848 (2017). https://doi.org/10.1007/s10008-017-3606-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-017-3606-3