Abstract
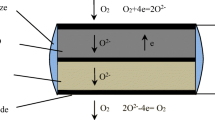
La0.8Sr0.2(Ga0.8Mg0.2)1-x Fe x O3-δ (LSGMF, x = 0.2–0.9) was prepared by a solid-state reaction method, and the characterization was investigated. Limiting current oxygen sensors were fabricated with La0.8Sr0.2Ga0.8Mg0.2O3-δ (LSGM) as solid electrolyte and La0.8Sr0.2(Ga0.8Mg0.2)0.1Fe0.9O3-δ (LSGMF9) as dense diffusion barrier. The influences of temperature, oxygen concentration, and the thickness of dense diffusion barrier (L) on sensing properties of oxygen sensors were investigated. The results show that the crystal structure of samples is perovskite. The electrical conductivity increases with increasing x. A transition from semiconductive to pseudometallic behavior which is associated with oxygen losses from the lattice is observed with an increase of temperature for x = 0.3, 0.5, and 0.7. The thermal expansion coefficient (TEC) in the temperature range 300–1000 °C increases to a maximum and then decreases with increasing x. TECs between LSGMF9 and LSGM are close. The limiting current oxygen sensor exhibits an excellent sensing performance, and the limiting current responses depend linearly on the oxygen concentration. As the L value increases, the limiting current decreases. The polarization resistance of the sensor decreases with increasing oxygen concentration.






Similar content being viewed by others
References
Garzon F, Raistrick I, Brosha E, Houlton R, Chung BW (1998) Dense diffusion barrier limiting current oxygen sensors. Sens Actuator B-Chem 50:125–130
Peng ZY, Liu ML, Balko E (2001) A new type of amperometric oxygen sensor based on a mixed-conducting composite membrane. Sens Actuator B-Chem 72:35–40
Liu T, Gao X, He BG, Yu JK (2016) A limiting current oxygen sensor based on LSGM as solid electrolyte and LSGMN (N = Fe, Co) as dense diffusion barrier. J Mater Eng Perform 25:2943–2950
Ishihara T, Matsuda H, Takita Y (1994) Doped LaGaO3 perovskite-type oxide as a new oxide ionic conductor. J Am Chem Soc 116:3801–3803
Trofimenko N, Ullmann H (1999) Transition metal doped lanthanum gallates. Solid State Ion 118:215–227
Chen FL, Liu ML (1998) Study of transition metal oxide doped LaGaO3 as electrode materials for LSGM-based solid oxide fuel cells. J Solid State Electr 3:7–14
Yi JY, Choi GM (2002) Phase characterization and electrical conductivity of LaSr(GaMg)1−x Mn x O3 system. Solid State Ion 148:557–565
Stevenson JW, Hasinska K, Canfield NL, Armstrong TR (2000) Influence of cobalt and iron additions on the electrical and thermal properties of (La,Sr)(Ga,Mg)O3−δ. J Electrochem Soc 147:3213–3218
Stevenson JW, Armstrong TR, Carneim RD, Pederson LR, Weber WJ (1996) Electrochemical properties of mixed conducting perovskites La1−x M x Coa1−y Fe3−δO (M = Sr, Ba, Ca). J Electrochem Soc 143:2722–2729
Politova ED, Aleksandrovskii VV, Kaleva GM, Mosunov AV, Suvorkin SV, Zaitsev SV, Sung JS, Choo KY, Kim TH (2006) Mixed conducting perovskite-like ceramics on the base of lanthanum gallate. Solid State Ion 177:1779–1783
Politova ED, Stefanovich SY, Avetisov AK, Aleksandrovskii VV, Glavatskih TY, Golubko NV, Kaleva GM, Mosunov AS, Venskovskii NU (2004) Processing, structure, microstructure, and transport properties of the oxygen-conductingceramics (La, Sr)(Ga, M)O y (M = Mg, Fe, Ni). J Solid State Electrochem 8:655–660
Kharton VV, Viskup AP, Yaremchenko AA, Baker RT, Gharbage GC, Mather GC, Figueiredo FM, Naumovich EN, Marques FMB (2000) Ionic conductivity of La(Sr)Ga(Mg, M)O3−δ (M = Ti, Cr, Fe, Co, Ni): effects of transition metal dopants. Solid State Ion 132:119–130
Kharton VV, Viskup AP, Naumovich EN, Lapchuk NM (1997) Mixed electronic and ionic conductivity of LaCo(M)O3 (M = Ga, Cr, Fe or Ni) I. oxygen transport in perovskites LaCoO3-LaGaO3. Solid State Ion 104:67–78
Usui T, Asada A, Nakazawa M, Osanai H (1989) Gas polarographic oxygen sensor using an oxygen/zirconia electrolyte. J Electrochem Soc 136:534–542
Nagao M, Kobayashi K, Yamamoto Y, Yamaguchi T, Oogushi A, Hibino T (2016) Rechargeable metal-air proton-exchange membrane batteries for renewable energy storage. ChemElectroChem 3:247–255
Kazuyo K, Masahiro N, and Takashi H (2016) A rechargeable tin air PEM battery using SnSO4 as an anode-active material. Chem Lett 45:161–163
Wagner C (1975) Equations for transport in solid oxides and sulfides of transition metals. Prog Solid State Chem 10:3–16
Takashi H, Kazuyo K, Masahiro N (2016) Kinetically driven switching and memory phenomena at the interface between a proton-conductive electrolyte and a titanium electrode. Sci Rep 6:31691
Muralidharan VS (1997) Warburg impedance-basics revisited. Anti-Corros Methods Mater 44:26–29
Meng FC, Xia T, Wang JP, Shi Z, Lian J, Zhao H, Bassat JM, Grenier JC (2014) Evaluation of layered perovskites YBa1–x Sr x Co2O5+δ as cathodes for intermediate-temperature solid oxide fuel cells. Int J Hydrog Energy 39:4531–4534
Acknowledgements
This work is financially supported by the National Natural Science Foundation of China (51374055, 50904016 and 52174059) and the Fundamental Research Funds for the Central Universities of China (N130502003).
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article is available at http://dx.doi.org/10.1007/s10008-017-3526-2.
Rights and permissions
About this article
Cite this article
Zhang, X., Liu, T., Yu, J. et al. A limiting current oxygen sensor with La0.8Sr0.2(Ga0.8Mg0.2)1-x Fe x O3-δ dense diffusion barrier. J Solid State Electrochem 21, 1323–1328 (2017). https://doi.org/10.1007/s10008-016-3486-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-016-3486-y