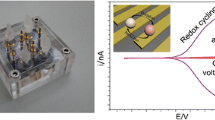
Abstract
High aspect ratio ultrathin (d < 10 nm) gold nanowires deposited on Si/SiO2 substrate are used as working electrodes for measuring cyclic voltammograms (CVs) in aqueous solutions of ferrocenemethanol and potassium hexacyanoferrate. The broadening of the peak separation as compared with that at a solid working electrode is explained as a result of the potential drop (“resistance overpotential”) along nanowires and nanowire network. The change in the CV shape over a sequence of scans is ascribed to a gradual breakup of individual nanowires and the respective transition of the linear diffusion to hemispherical diffusion regularities.

ᅟ





Similar content being viewed by others
References
Halder A, Ravishankar N (2007) Ultrafine single-crystalline gold nanowire arrays by oriented attachment. Adv Mater 19:1854–1858
Kisner A, Heggen M, Fernández E, Lenk S, Mayer D, Simon U, Offenhäusser A, Mourzina Y (2011) The role of oxidative etching in the synthesis of ultrathin single crystalline Au nanowires. Chem–A Europ J 17:9503–9507
Roy A, Pandey T, Ravishankar N, Singh AK (2013) Single crystalline ultrathin gold nanowires: promising nanoscale interconnects. AIP Adv 3:032131-1–032131-7
Kisner A, Heggen M, Mayer D, Simon U, Offenhäusser A, Mourzina Y (2014) Probing the effect of surface chemistry on the electrical properties of ultrathin gold nanowire sensors. Nanoscale 6:5146–5155
Liu J, Fu TM, Cheng Z, Hong G, Zhou T, Jin L, Duvvuri M, Jiang Z, Kruskal P, Xie C, Suo Z, Fang Y, Lieber CM (2015) Syringe-injectable electronics. Nat Nanotechnol 10:629–636. doi:10.1038/nnano.2015.115
Pud S, Kisner A, Heggen M, Belaineh D, Temirov R, Simon U, Offenhäusser A, Mourzina Y, Vitusevich S (2013) Features of transport in ultrathin gold nanowire structures. Small 9:846–852
Guerin H, Yoshihira M, Kura H, Ogawa T, Sato T, Maki H (2012) Coulomb blockade phenomenon in ultra-thin gold nanowires. J Appl Phys 111:054304-1–054304-4
Loubat A, Escoffier W, Lacroix LM, Viau G, Tan R, Carrey J, Warot-Fonrose B, Raquet B (2013) Cotunneling transport in ultra-narrow gold nanowire bundles. Nano Res 6:644–651
Chandni U, Kundu P, Singh AK, Ravishankar N, Ghosh A (2011) Insulating state and breakdown of Fermi liquid description in molecular-scale single-crystalline wires of gold. ACS Nano 5:8398–8403
Lacroix LM, Arenal R, Viau G (2014) Dynamic HAADF-STEM observation of a single-atom chain as the transient state of gold ultrathin nanowire breakdown. J Am Chem Soc 136:13075–13077
Roy A, Pandey T, Ravishankar N, Singh AK (2014) Semiconductor-like sensitivity in metallic ultrathin gold nanowire-based sensors. J Phys Chem C 118:18676–18682
Koposova E, Kisner A, Shumilova G, Ermolenko Y, Offenhäusser A, Mourzina Y (2013) Oleylamine-stabilized gold nanostructures for bioelectronic assembly direct electrochemistry of cytochrome c. J Phys Chem C 117:13944–13951
Xia BY, Wu HB, Yan Y, Lou XW, Wang X (2013) Ultrathin and ultralong single-crystal platinum nanowire assemblies with highly stable electrocatalytic activity. J Am Chem Soc 135:9480–9485
Koposova E, Liu X, Kisner A, Ermolenko Y, Shumilova G, Offenhäusser A, Mourzina Y (2014) Bioelectrochemical systems with oleylamine-stabilized gold nanostructures and horseradish peroxidase for hydrogen peroxide sensor. Biosens Bioelectron 57:54–58
Langley DP, Lagrange M, Giusti G, Jiménez C, Bréchet Y, Nguyen ND, Bellet D (2014) Metallic nanowire networks: effects of thermal annealing on electrical resistance. Nanoscale 6:13535–13543
Tyagi P, Postetter D, Saragnese DL, Randall CL, Mirski MA, Gracias DH (2009) Patternable nanowire sensors for electrochemical recording of dopamine. Anal Chem 81:9979–9984
Dickinson T, Sutton PR (1974) The study of adsorption by measurement of electrode resistance. Electrochim Acta 19:427–435
Fuchs K (1938) The conductivity of thin metallic films according to the electron theory of metals. Proc Camb Philos Soc 34:100–108
Khaligh HH, Goldthorpe IA (2013) Failure of silver nanowire transparent electrodes under current flow. Nanoscale Res Lett 8:235–231
Duan BK, Zhang J, Bohn PW (2012) Conductance-based chemical sensing in metallic nanowires and metal–semiconductor nanostructures. Anal Chem 84:2–8
Liu Z, Searson PC (2006) Single nanoporous gold nanowire sensor. J Phys Chem B 110:4318–4322
Hung D, Liu Z, Shah N, Hao Y, Searson PC (2007) Finite size effects in ordered macroporous electrodes fabricated by electrodeposition into colloidal crystal templates. J Phys Chem C 111:3308–3313
He H, Tao N (2002) Interactions of molecules with metallic quantum wires. Adv Mater 14:161–164
Li SJ, Du JM, Chen J, Mao NN, Zhang MJ, Pang H (2014) Electrodeposition of cobalt oxide nanoparticles on reduced graphene oxide: a two-dimensional hybrid for enzyme-free glucose sensing. J Solid State Electrochem 18:1049–1056
Radhakrishnan S, Siju CR, Mahanta D, Patil S, Madras G (2009) Conducting polyaniline–nano-TiO2 composites for smart corrosion resistant coatings. Electrochim Acta 54:1249–1254
Wang Q, Zhang Y, Ye W, Wang C (2016) Ni(OH)2/MoSx nanocomposite electrodeposited on a flexible CNT/PI membrane as an electrochemical glucose sensor: the synergistic effect of Ni(OH)2 and MoSx. J Solid State Electrochem 20:133–142
Scanlon MD, Peljo P, Mendez MA, Smirnov E, Girault HH (2015) Charging and discharging at the nanoscale: Fermi level equilibration of metallic nanoparticles. Chem Sci 6:2705–2720
Nikolaev K, Ermakov S, Ermolenko Y, Averyaskina E, Offenhäusser A, Mourzina Y (2015) A novel bioelectrochemical interface based on in situ synthesis of gold nanostructures on electrode surfaces and surface activation by Meerwein’s salt. A bioelectrochemical sensor for glucose determination. Bioelectrochem 105:34–43
Song JH, Wu Y, Messer B, Kind P, Yang P (2001) Metal nanowire formation using Mo3Se3 − as reducing and sacrificing templates. J Am Chem Soc 123:10397–10398
Bowden FP, Agar JN (1938) General and physical chemistry. 5: irreversible electrode process. Ann Rep Chem Soc 35:90–113
Scholz F (ed) (2002) Electroanalytical methods. Springer, Berlin, Heidelberg
Brown ER, Smith DE, Booman GL (1968) A study of operational amplifier potentiostats employing positive feedback for IR compensation 1. Theoretical analysis of stability and bandpass characteristics. Anal Chem 40:1411–1423
Feldberg SW (2008) Effect of uncompensated resistance on the cyclic voltammetric response of an electrochemically reversible surface-attached redox couple: uniform current and potential across the electrode surface. J Electroanal Chem 624:45–51
Fangohr H, Chernyshenko DS, Franchin M, Fischbacher T, Meier G (2011) Joule heating in nanowires. Phys Rev B 84:054437-1–054437-12
Popp E (2010) Energy dissipation and transport in nanoscale devices. Nano Res 3:147–169
Cannes C, Kanoufi F, Bard AJ (2003) Cyclic voltammetry and scanning electrochemical microscopy of ferrocenemethanol at monolayer and bilayer-modified gold electrodes. J Electroanal Chem 547:83–91
Marecek V, Samec Z, Weber J (1978) The dependence of the electrochemical charge-transfer coefficient on the electrode potential. Study of the Fe(CN)3− 6/Fe(CN)4− 6 redox reaction on polycrystalline Au electrode in KF solutions. J Electroanal Chem 94:169–185
Bourdillon C, Demaille C, Moiroux J, Saveant JM (1995) Catalysis and mass transport in spatially ordered enzyme assemblies on electrodes. J Am Chem Soc 117:11499–11506
Swaddle TW (2005) Homogeneous versus heterogeneous self-exchange electron transfer reactions of metal complexes: insights from pressure effects. Chem Rev 105:2573–2608
Dogonadze RR, Ulstrup J, Kharkats YI (1972) A theory of electrode reactions through bridge transition states; bridges with a discrete electronic spectrum. J Electroanal Chem 39:47–61
Krulic D, Fatouros N, Khoshtariya DE (1998) Kinetic data for the hexacyanoferrate (II)/(III) couple on platinum electrode in various chlorides of monovalent cations. J Chim Phys 95:497–512
Scharifker BR (1988) Diffusion to ensembles of microelectrodes. J Electroanal Chem 240:61–76
Boo H, Jeong RA, Park S, Kim KS, An KH, Lee YH, Han JH, Kim HC, Chung TD (2006) Electrochemical nanoneedle biosensor based on multiwall carbon nanotube. Anal Chem 78:617–620
Li Y, Wu Q, Jiao S, Xu C, Wang L (2013) Single Pt nanowire electrode: preparation, electrochemistry, and electrocatalysis. Anal Chem 85:4135–4140
Kleijn SEF, Lai SCS, Koper MTM, Unwin PR (2014) Electrochemistry of nanoparticles. Angew Chem Int Ed 53:3558–3586
Acknowledgments
Ms. Hannah Freyer is gratefully acknowledged for improving the English of the paper. Dr. G. Panaitov is gratefully acknowledged for the helpful discussions. Financial support from the Russian Foundation for Basic Research (RFBR) grant 14-03-01079 for studies on the nanostructure synthesis and St. Petersburg State University grant 12.38.218.2015 for the electrochemical studies are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
K.N. Mikhelson is ISE member.
Rights and permissions
About this article
Cite this article
Muratova, I.S., Mikhelson, K.N., Ermolenko, Y. et al. On “resistance overpotential” caused by a potential drop along the ultrathin high aspect ratio gold nanowire electrodes in cyclic voltammetry. J Solid State Electrochem 20, 3359–3365 (2016). https://doi.org/10.1007/s10008-016-3280-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-016-3280-x