Abstract
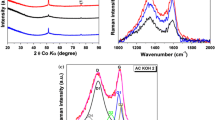
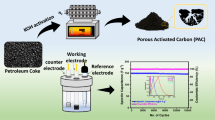
In this paper, cabbage leaves (CLs) were used to synthesize porous activated carbon by the carbonization and activation processes. The material for CLs were carbonized at 600 °C and activated at 800 °C with the KOH/C-600 mass ratio 4 (denoted as AC-800) show typical amorphous character and display porous structures with high specific surface areas 3102 m2/g via XRD and BET measurements. As the electro-active material, AC-800 electrode exhibit ideal capacitive behaviors in aqueous electrolytes and the maximal specific capacitance is as high as 336 F/g at the current density of 1 A/g. Furthermore, AC-800 electrode shows excellent electrochemical cycle stability with ∼95 % initial capacitance being retained after 2000 cycles. The desirable capacitive performances enable the CLs to act as a new biomass source of carbonaceous materials for high-performance supercapacitors and low-cost electrical energy storage devices.







Similar content being viewed by others
References
Zhao L, Fan L, Zhou M, Guan H, Qiao S, Antonietti M, Titirici M-M (2010) Nitrogen-containing hydrothermal carbons with superior performance in supercapacitors. Adv Mater 22:5202–5206
Zhai Y, Dou Y, Zhao D, Fulvio PF, Mayes RT, Dai S (2009) Carbon materials for chemical capacitive energy storage. Adv Mater 23:4828–4850.
Liu C, Li F, Ma L, Cheng H (2010) Advanced materials for energy storage. Adv Mater 22:E28–E62.
Xu B, Wu F, Su Y, Cao G, Chen S, Zhou Z, Yang Y (2008) Competitive effect of KOH activation on the electrochemical performances of carbon nanotubes for EDLC: balance between porosity and conductivity. Electron Acta 53:7730–7735.
Zhang H, Cao G, Yang Y (2009) Carbon nanotube arrays and their composites for electrochemical capacitors and lithium-ion batteries. Energy Environ Sci 2:932–943.
Béguin F, Presser V, Balducci A, Frackowiak E (2014) Carbons and electrolytes for advanced supercapacitors. Adv Mater 26:2219–2251.
Simon P, Gogotsi Y (2008) Materials for electrochemical capacitors. Nat Mater 7:845–854.
Jänes A, Kurig H, Lust E (2007) Characterisation of activated nanoporous carbon for supercapacitor electrode materials. Carbon 45:1226–1233.
Laheäär A, Jänes A, Lust E (2011) Electrochemical properties of carbide-derived carbon electrodes in non-aqueous electrolytes based on different Li-salts. Electron Acta 56:9048–9055.
Pandolfo AG, Hollenkamp AF (2006) Carbon properties and their role in supercapacitors. J Power Sources 157:11–27.
Zhu YW, Murali S, Stoller MD, Ganesh KJ, Cai WW, Ferreira PJ, Pirkle A, Wallace RM, Cychosz KA, Thommes M, Su D, Stach EA, Ruoff RS (2011) Carbon-Based supercapacitors produced by activation of graphene. Science 332:1537–1541.
Wang HL, Gao QM, Hu J (2010) Preparation of porous doped carbons and the high performance in electrochemical capacitors. Microporous Mesoporous Mater 131:89–96.
Jurcakova DH, Kodama M, Shiraishi S, Hatori H, Zhu ZH, Lu GQ (2009) Nitrogen-enriched nonporous carbon electrodes with extraordinary supercapacitance. Adv Funct Mater 19:1800–1809.
Górka J, Jaroniec M (2011) Hierarchically porous phenolic resin-based carbons obtained by block copolymer-colloidal silica templating and post-synthesis activation with carbon dioxide and water vapor. Carbon 49:154–160.
Plaza MG, Pevida C, Martín CF, Fermoso J, Pis JJ, Rubiera F (2010) Developing almond shell-derived activated carbons as CO2 adsorbents. Sep Purif Technol 71:102–106.
Raymundo-Piñero E, Azaïsa P, Cacciaguerra T, Cazorla-Amorósb D, Linares-Solanob A, Béguin F (2005) KOH and NaOH activation mechanisms of multiwalled carbon nanotubes with different structural organisation. Carbon 43:786–795.
Tee E, Tallo I, Kurig H, Thomberg T, Jänes A, Lust E (2015) Huge enhancement of energy storage capacity and power density of supercapacitors based on the carbon dioxide activated microporous SiC-CDC. Electron Acta 161:364–370.
Lillo-Ródenas MA, Cazorla-Amorós D, Linares-Solano A (2003) Understanding chemical reactions between carbons and NaOH and KOH: an insight into the chemical activation mechanism. Carbon 41:267–275.
Wang R, Wang P, Yan X, Lang J, Peng C, Xue Q (2012) Promising porous carbon derived from celtuce leaves with outstanding supercapacitance and CO2 capture performance. ACS Appl Mater Interfaces 4:5800–5806
Peng C, Yan X, Wang R, Lang J, Ou Y, Xue Q (2013) Promising activated carbons derived from waste tea-leaves and their application in high performance supercapacitors electrodes. Electron Acta 87:401–408
Sing KSW, Everett DH, Haul RAW, Moscou L, Pieroti RA, Rouquerol J, Siemieniewska T (1985) Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl Chem 57:603–619
Liu H, Wang K, Teng H (2005) A simplified preparation of mesoporous carbon and the examination of the carbon accessibility for electric double layer formation. Carbon 43:559–566
Alhamed YA, Bamufleh HS (2009) Sulfur removal from model diesel fuel using granular activated carbon from dates’ stones activated by ZnCl2. Fuel 88:87–94
Hsieh C, Lin Y (2006) Synthesis of mesoporous carbon composite and its electric double-layer formation behavior. Microporous Mesoporous Mater 93:232–239
Wang K, Teng H (2006) The performance of electric double layer capacitors using particulate porous carbons derived from PAN fiber and phenol-formaldehyde resin. Carbon 44:3218–3225
Tang SB, Lai MO, Lu L (2008) Li-ion diffusion in highly (0 0 3) oriented LiCoO2 thin film cathode prepared by pulsed laser deposition. J Alloys Compd 449:300–303
Wang WH, Wang XD (2007) Investigation of Ir-modified carbon felt as the positive electrode of an all-vanadium redox flow battery. Electron Acta 52:6755–6762
Xiang X, Liu E, Huang Z, Shen H, Tian Y, Xiao C, Yang J, Mao Z (2011) Preparation of activated carbon from polyaniline by zinc chloride activation as supercapacitor electrodes. J Solid State Electrochem 15:2667–2674
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant No. 51462031), the Fundamental Research Funds for the Central Universities (Grant No. 31920150010), the National Natural Science Foundation of Gansu Province (Grant Nos. 145RJZA218 and 1308RJZA129), and Gansu Provincial Department of education project (Grant No. 2014B-008). This work was also supported by the Introduction of Talent project of Northwest University for Nationality (Grant No. xbmuyjrc201307).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, P., Wang, Q., Zhang, G. et al. Promising activated carbons derived from cabbage leaves and their application in high-performance supercapacitors electrodes. J Solid State Electrochem 20, 319–325 (2016). https://doi.org/10.1007/s10008-015-3042-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-015-3042-1