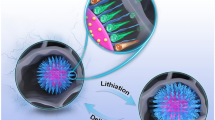
Abstract
We report a novel composite, sulfur (S) encapsulated in porous carbon nanospheres (PCNS) and coated with conductive polyaniline (PANI) (PCNS-S@PANI), as cathode of lithium–sulfur battery. PCNS is prepared by convenient and controllable hydrothermal synthetic route and loaded with S via chemical deposition and then coated with conductive polyaniline via in situ polymerization under the control of ascorbic acid. The physical and electrochemical performances of the resulting PCNS-S@PANI are investigated by scanning electron microscopy, X-ray powder diffraction, X-ray photoelectron spectroscopy, nitrogen adsorption–desorption isotherms, thermogravimetric analysis, electronic conductivity measurement, galvanostatic charge–discharge test, and electrochemical impedance spectroscopy. It is found that PCNS-S@PANI exhibits excellent charge–discharge performances as cathode of lithium–sulfur battery: delivering a discharge capacity of 881 mAh g−1 at 0.2 C (1 C = 1672 mA g−1) with a capacity retention of 72 % after 100 cycles and a rate capacity of 324 mAh g−1 at 2 C. These natures can be attributed to the co-contribution of PCNS and conductive PANI to the improvement in electronic conductivity and chemical stability of sulfur cathode.








Similar content being viewed by others
References
Ji XL, Lee KT, Nazar LF (2009) A highly ordered nanostructured carbon-sulfur cathode for lithium-sulfur batteries. Nat Mater 8(6):500–506
Yang Y, Zheng GY, Cui Y (2013) Nanostructured sulfur cathodes, Chemical Society Reviews. 42(7):3018–3032
Bruce PG, Freunberger SA, Hardwick LJ, Tarascon JM (2012) Li-O2 and Li-S batteries with high energy storage. Nat Mater 11(1):19–29
Yang Y, Zheng GY, Misra S, Nelson J, Toney MF, Cui Y (2012) High-Capacity Micrometer-Sized Li2S Particles as Cathode Materials for Advanced Rechargeable Lithium-Ion Batteries. J Am Chem Soc 134(37):15387–15394
Joongpyo S, Striebel KA, Cairns EJ (2002) The Lithium/Sulfur Rechargeable Cell: Effects of Electrode Composition and Solvent on Cell Performance. J Electrochem Soc 149(10):A1321–A1325
Hagena M, Dörfler S, Althues H, Tübke J, Hoffmann MJ, Kaskel S, Pinkwart K (2012) Lithium-sulphur batteries-binder free carbon nanotubes electrode examined with various electrolytes. J Power Sources 213:239–248
Wei W, Wang JL, Zhou LJ, Yang J, Schumann B, NuLi Y (2011) CNT enhanced sulfur composite cathode material for high rate lithium battery. Electrochem Commun 13:399–402
Huang JQ, Zhang Q, Zhang SM, Liua XF, Zhu WC, Qian WZ, Wei F (2013) Aligned sulfur-coated carbon nanotubes with a polyethylene glycol barrier at one end for use as a high efficiency sulfur cathode. Carbon 58:99–106
Guo JC, Xu YH, Wang CS (2011) Sulfur-Impregnated Disordered Carbon Nanotubes Cathode for Lithium-Sulfur Batteries. Nano Lett 11:4288–4294
Ji LW, Rao MM, Aloni S, Wang L, Cairns EJ, Zhang YG (2011) Porous carbon nanofiber-sulfur composite electrodes for lithium/sulfur cells. Energy Environ Sci 4:5053–5059
Rao MM, Song XY, Cairns EJ (2012) Nano-carbon/sulfur composite cathode materials with carbon nanofiber as electrical conductor for advanced secondary lithium/sulfur cells. J Power Sources 205:474–478
Li XG, Rao MM, Chen DR, Lin HB, Liu YL, Liao YH, Xing LD, Li WS (2015) Sulfur supported by carbon nanotubes and coated with polyaniline: Preparation and performance as cathode of lithium-sulfur cell. Electrochim Acta 166:93–99
Li NW, Zheng MB, Lu HL, Hu ZB, Shen CF, Chang XF, Ji JB, Cao JM, Shi Y (2012) High-rate lithium-sulfur batteries promoted by reduced graphene oxide coating. Chem Commun 48:4106–4108
Yu MP, Yuan WJ, Li C, Hong JD, Shi GQ (2014) Performance enhancement of graphene-sulfur composite in lithium-sulfur battery by coating an ultrathin Al2O3 film via atomic layer deposition. J Mater Chem A 2:7360–7366
Ji LW, Rao MM, Zheng HM, Zhang L, Li YC, Duan WH, Guo JH, Cairns EJ, Zhang YG (2011) Graphene Oxide as a Sulfur Immobilizer in High Performance Lithium/Sulfur Cells. J Am Chem Soc 133:18522–18525
Wang M, Zhang HM, Zhang YN, Li J, Zhang FX, Hu W (2013) Composite of sulfur impregnated in porous hollow carbon spheres as the cathode of Li-S batteries with high performance. J Solid State Electrochem 17:2243–2250
Jayaprakash N, Shen J, Moganty SS, Corona A, Archer LA (2011) Porous Hollow Carbon@Sulfur Composites for High-Power Lithium-Sulfur Batteries. Angew Chem Int Ed 123:6026–6030
Sun XM, Li YD (2004) Colloidal Carbon Spheres and Their Core/Shell Structures with Noble-Metal Nanoparticles. Angew Chem Int Ed 43:597–601
Sun XM, Li YD (2004) Ga2O3 and GaN Semiconductor Hollow Spheres. Angew Chem Int Ed 43:3827–3831
Titirici MM, Antonietti M, Thomas A (2006) A Generalized Synthesis of Metal Oxide Hollow Spheres Using a Hydrothermal Approach. Chem Mater 18:3808–3812
Yang MS, Zhang GX, Wu CY, Chang Z, Sun XM, Duan X (2012) Preparation of Multi-Metal Oxide Hollow Sphere Using Layered Double Hydroxide Precursors. Chin J Chem 30:2183–2188
Chang BB, Guan DX, Tian XL, Yang ZC, Dong XP (2013) Convenient synthesis of porous carbon nanospheres with tunable pore structure and excellent adsorption capacity. J Hazard Mater 262:256–264
Yin LC, Wang JL, Lin FJ, Yang J, Nuli Y (2012) Polyacrylonitrile/graphene composite as a precursor to a sulfur-based cathode material for high-rate rechargeable Li-S batteries. Energy Environ Sci 5:6966–6972
Liang X, Wen ZY, Liu Y, Zhang H, Jin J, Wu MF, Wu XW (2012) A composite of sulfur and polypyrrole-multi walled carbon combinatorial nanotube as cathode for Li/S battery. J Power Sources 206:409–413
Li L, Ruan GD, Peng ZW, Yang Y, Fei HL, Raji AQ, Samuel ELG, Tour JM (2014) Enhanced Cycling Stability of Lithium Sulfur Batteries Using Sulfur-Polyaniline-Graphene Nanoribbon Composite Cathodes. ACS Appl Mater Interfaces 6:15033–15039
Zhao XH, Kim JK, Ahn KJ, Cho KK, Ahn JH (2013) A ternary sulfur/polyaniline/carbon composite as cathode material for lithium sulfur batteries. Electrochim Acta 109:145–152
Wu F, Chen JZ, Li L, Zhao T, Chen RJ (2011) Improvement of Rate and Cycle Performence by Rapid Polyaniline Coating of a MWCNT/Sulfur Cathode. J Phys Chem C 115:24411–24417
Hu ZH, Srinivasan MP, Ni YM (2001) Novel activation process for preparing highly microporous and mesoporous activated carbons. Carbon 39:877–886
Yue ZR, Mangun CL, Economy J (2002) Preparation of fibrous porous materials by chemical activation 1. ZnCl2 activation of polymer-coated fibers. Carbon 40:1181–1191
Wang CH, Chen HW, Dong WL, Ge J, Lu W, Wu XD, Guo L, Chen LW (2014) Sulfur-amine chemistry-based synthesis of multiwalled carbon nanotube–sulfur composites for high performance Li-S batteries. Chem Commun 20:1202–1204
Li YX, Li WY, Zhou HP, Wang FY, Chen Y, Wang Y, Yu C (2015) A facile method for the sensing of antioxidants based on the redox transformation of polyaniline. Sens Actuators B 208:30–35
Geng XY, Rao MM, Li XP, Li WS (2013) Highly dispersed sulfur in multi-walled carbon nanotubes for lithium/sulfur battery. J Solid State Electrochem 17:987–992
Zhou GM, Zhao YB, Manthiram (2015) Dual-confined flexible sulfur cathodes encapsulated in nitrogen-doped double-shelled hollow carbon spheres and wrapped with grapheme for Li-S batteries. Adv Energy Mater. doi: 10.1002/aenm.201402263
Wang C, Wan W, Chen JT, Zhou HH, Zhang XX, Yuan LX, Huang YH (2013) Dual core-shell structured sulfur cathode composite synthesized by a one-pot route for lithium sulfur batteries. J Mater Chem A 1(5):1716–1723
Zhou GM, Wang DW, Li F, Hou PX, Yin LC, Liu C, Lu GQ(M), Gentle IR, Cheng HM (2012) A flexible nanostructured sulphur-carbon nanotube cathode with high rate performance for Li-S batteries. Energy Environ Sci 5:8901–8906
Lu DS, Li WS, Zuo XX, Yuan ZZ, Huang QM (2007) A Study on Kinetics of Li+ Insertion/Desertion in LixMn2O4 (0≤x≤1) by Electrochemical Impedance Spectroscopy. J Phys Chem C 111:12067–12074
Songa J, Bazant MZ (2013) Effects of Nanoparticle Geometry and Size Distribution on Diffusion Impedance of Battery Electrodes. J Electrochem Soc 160(1):A15–A24
Ciucci F, Lai W (2012) Electrochemical impedance spectroscopy of phase transition materials. Electrochim Acta 81:205–216
Lai W, Ciucci F (2011) Small-Signal Apparent Diffusion Impedance of Intercalation Battery Electrodes. J Electrochem Soc 158(2):A115–A121
Acknowledgments
The authors are highly grateful for the financial support from the joint project of National Natural Science Foundation of China and Natural Science Foundation of Guangdong Province (Grant No. U1401248), Natural Science Foundation of Guangdong Province (Grant No. S2013040016471), China Postdoctoral Science Foundation funded project (Grant Nos. 2013M530369 and 2014T70819), the key project of Science and Technology in Guangdong Province (Grant Nos. 2012A090300012 and 2013B090800013), and the scientific research project of Department of Education of Guangdong Province (Grant No. 2013CXZDA013).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, X., Rao, M. & Li, W. Sulfur encapsulated in porous carbon nanospheres and coated with conductive polyaniline as cathode of lithium–sulfur battery. J Solid State Electrochem 20, 153–161 (2016). https://doi.org/10.1007/s10008-015-3013-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-015-3013-6