Abstract
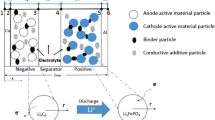
A model is proposed for the description of the discharging of porous graphite electrodes. The model takes into account the nonequivalence of the different layers of the internal porous surface, as well as change in the potential and current density in these layers depending on the amount of passed charge. The model uses a special “algorithmic approach” for calculating current distribution in such a non-stationary system, based on consistent computer formulation and solution of Kirchhoff’s algebraic equations for the equivalent electrical circuit that simulates a porous electrode with the given thickness on different time intervals. The analysis of the model enables a better understanding of the mechanism of current generation in porous graphite electrodes and offers and explanation of the effect of electrochemical process penetration into the thick electrodes during the discharge process. It is shown, particularly, that classical almost exponential current distributions in the initial quasi-stationary period of time changes considerably during the further discharge process. After some period of discharge, the current peaks originate in the electrode. These discharge currents travel into the depth of the electrode, involving again and again new layers the porous electrode. The effect of some design and technological parameters (like the electrode thickness, current density, resistor of separator, polymer binders, etc.) has been analyzed. This gives a possibility to estimate the influence of such parameters on the discharge curves and operating characteristics of lithium-ion batteries and hybrid electrochemical capacitors with negative graphite electrode.









Similar content being viewed by others
References
Chen J (2013) Recent progress in advanced materials for lithium ion batteries. Materials 6:56–183
Aifantis K, Hackney S, Kumar R (2010) eds. High Energy Density Lithium Batteries. Wiley-VCH Verlag GmbH & Co., Weinheim
Bagotsky VS, Skundin AM, Volfkovich YM (2015) Electrochemical Power Sources: Batteries, Fuel Cells, and Supercapacitors. Wiley, New York
Khomenko V, Raymundo-Piñero E, Béguin F (2008) High-energy density graphite/AC capacitor in organic electrolyte. J Power Sources 177(2):643–651
Decaux C, Lota G, Raymundo-Piñero E, Frackowiak E, Béguin F (2012) Electrochemical performance of a hybrid lithium-ion capacitor with a graphite anode preloaded from lithium bis(trifluoromethane)sulfonimide-based electrolyte. Electrochim Acta 86:282–286
Daniel-Beck VS (1948) On polarization of porous electrodes. I. On distribution of current and potential within the electrode (in Russian). Rus J Phys Chem 22(6):697–710
Frumkin AN (1949) Cathode process distribution in the semi-infinity tube hollow (in Russian). Rus J Phys Chem 23(12):1477–1482
Newman IJ, Tobias SW (1962) Theoretical analysis of current distribution in porous electrodes. J Electrochem Soc 109(12):1183–1191
Ksenzhek OS (1964) Macrokinetics of processes on porous electrodes. Electrochim Acta 9(9):629–637
Micka K, Rousar T (1974) Theory of porous electrodes. Electrochim Acta 19(8):499–502
Barsukov VZ, Ksenzhek OS, Erpert AM, Sagoyan LN (1974) Distribution of electrochemical process in the depth of sintered nickel-hydroxide electrode (in Russian). Rus J Electrochemistry 10(2):237–243
Barsukov V (1980) Design and application of mathematical and physical modeling methods of electrode process dynamics for optimal construction of electrochemical power sources. 31st ISE Meeting Venice, Extended Abstracts (2):920–922. 31st ISE Meeting, Venice, Italy, Sept 22-26, 1980 Extended Abstract, Volume 2, Edted by Enrico Vecchi, Institute of Polarography and Preparative Electrochemistry of the National Research Council - Padua
Barsukov V (1981) Methods of macrokinetics simulation, calculation and optimization of electrochemical systems with distributed parameters under the conditions of limited data about the mechanism and kinetics of electrode processes. 32nd ISE Meeting Dubrovnik, Extended Abstracts (1):1093–1096. 32nd ISE Meeting, Dubrovnik/Cavtat, Yugoslavia, Sept. 13-20, 1981 Extended Abstracts Volume IIDivisions I, II, III, IV, V, VI, VII
Zheng T, Reimers JN, Dahn JR (1995) The effect of turbostratic disorder in graphitic carbons on the intercalation of lithium. Phys Rev B 51:734–741
Center for Electrical Energy Storage: tailored interfaces energy frontier research center (2012) US Argonne Nat Lab http://www.cse.anl.gov/cees/ Accessed June 2012
Barsukov VZ, Rogoza BE, Sagoyan LN (1984) Modeling the discharge process in a grain of active material for the nickel-hydroxide electrode (in Russian). Rus J Electrochemistry 20(12):1631–1635
Barsukov V (1997) Makrokinetics of process during the discharge of nickel hydroxide electrode. European Workshop on Current and Potential Distributions in Complex Electrochemical Systems, Nancy Abstracts:3-4
Vorotyntsev MA, Rubashkin AA, Badiali JP (1996) Potential distribution across the electroactive-polymer film between the metal and solution as a function of the film charging level. Electrochim Acta 41(14):2313–2320
Vorotyntsev MA, Vieil E, Heinze J (1996) Charging-discharging process of polypyrrole films in solutions of tetraphenylborate anions. In: Barsukov VZ, Beck F (eds) New Promising Electrochemical Systems for Rechargeable Batteries. Kluwer Academic Publishers, Dordrecht
Vorotyntsev MA, Deslouis C, Musiani MM, Tribollet B, Aoki K (1999) Transport across an electroactive-polymer film in contact with media allowing both ionic and electronic interfacial exchange. Electrochim Acta 44(12):2105–2115
Barsukov VZ, Khomenko VG, Chivikov SV et al (2001) On the faradaic and non-faradaic mechanisms of electrochemical processes in conducting polymers and some other reversible systems with solid-phase reagents. Electrochim Acta 46(26–27):4083–4094
Khomenko V, Barsukov V, Katashinskii A (2005) The catalytic activity of conducting polymers toward oxygen reduction. Electrochim Acta 50(7–8):1675–1683
Acknowledgment
Authors acknowledge the European Commission for the financial support of these researches in the framework of the FP7 “Energy Caps” project.
Author information
Authors and Affiliations
Corresponding author
Additional information
The paper is dedicated to Prof. Vorotyntsev’s jubilee.
Rights and permissions
About this article
Cite this article
Barsukov, V., Langouche, F., Khomenko, V. et al. Modeling of porous graphite electrodes of hybride electrochemical capacitors and lithium-ion batteries. J Solid State Electrochem 19, 2723–2732 (2015). https://doi.org/10.1007/s10008-015-2835-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-015-2835-6