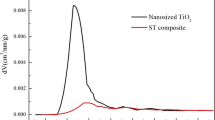
Abstract
Titania–sulfur (TiO2–S) composite cathode materials were synthesized for lithium–sulfur batteries. The composites were characterized and examined by X-ray diffraction, nitrogen adsorption/desorption measurements, scanning electron microscopy, and electrochemical methods, such as cyclic voltammetry, electrochemical impedance spectroscopy, and galvanostatic charge–discharge tests. It is found that the mesoporous TiO2 and sulfur particles are uniformly distributed in the composite after a melt-diffusion process. When evaluating the electrochemical properties of as-prepared TiO2–S composite as cathode materials in lithium–sulfur batteries, it exhibits much improved cyclical stability and high rate performance. The results showed that an initial discharge specific capacity of 1,460 mAh/g at 0.2 C and capacity retention ratio of 46.6 % over 100 cycles of composite cathode, which are higher than that of pristine sulfur. The improvements of electrochemical performances were due to the good dispersion of sulfur in the pores of TiO2 particles and the excellent adsorbing effect on polysulfides of TiO2.









Similar content being viewed by others
References
Dharmasena P, Stuart L (1993) Science 261:1029–1032
Bruce PG, Freunberger SA, Hardwick LJ, Tarascon JM (2012) Nat Mater 11:19–29
Ji XL, Nazar LF (2010) J Mater Chem 20:9821–9826
Cheon SE, Ko KS, Cho JH, Kim SW, Chin EY, Kim HT (2003) J Electrochem Soc 150:A796–799
Cheon SE, Ko KS, Cho JH, Kim SW, Chin EY, Kim HT (2003) J Electrochem Soc 150:A800–A805
Elazari R, Salitra G, Talyosef Y, Grinblat J, Charislea SK, Xiao A, Affinito J, Aurbach D (2010) J Electrochem Soc 157:A1131–1138
Mikhaylik YV, Akridge JR (2004) J Electrochem Soc 151:A1969–A1976
Barchasz C, Leprêtreb JC, Alloinb F, Patoux S (2012) J Power Sources 199:322–330
Gorkovenko A, Skotheim TA, Xu ZS (2005) US Patent No 6878488
Zheng W, Liu YW, Hu XG, Zhang CF (2006) Electrochimica Acta 51:1330–1335
Ji XL, Evers S, Black R, Nazar LF (2011) Nat Commun 2:325. doi:10.1038/ncomms1293
Zhang YG, Bakenov Z, Zhao Y, Konarov A, Doan TNL, Sun KEK, Yermukhambetova A, Chen P (2013) Powder Technol 235:248–255
Zhang YG, Zhao Y, Yermukhambetova A, Bakenov Z, Chen P (2013) J Mater Chem 1:A295–A301
Zhang Y, Wu XB, Feng H, Wang LZ, Zhang AQ, Xia TC, Dong HC (2009) Int J Hydrogen Energy 34:1556–1559
Choi YJ, Jung BS, Lee DJ, Jeong JH, Kim KW, Ahn HJ, Cho KK, Gu HB (2007) Phys Scr T129:62–65
Kang D, Sheng PW, Hanyu Z, Wu JP (2013) Mater Res Bull 48:2079–2083
Zheng W, Hu XG, Zhang CF (2006) Electrochem Solid State Lett 9:A364–A367
Scott E, Taeeun Y, Nazar LF (2012) J Phys Chem C 116:19653–19658
Zhi WS, Li WY, Judy JC, Zheng GG, Yuan Y, Matthew TM, Po CH, Cui Y (2013) Nat Commun 4:1331–1336
Vijayalakshmi R, Rajendran V (2012) Arch Appl Sci Res 4:1183–1190
Wu MS, Lee JT, Chiang PC, Lin JC (2007) J Mater Sci 42:259–265
Deng ZF, Zhang ZA, Lai YQ, Liu J, Liu YX, Li J (2013) Solid State Ionics 238:44–49
Zhang CF, Wu HB, Yu CZ, Guo ZP, Lou WX (2012) Angew Chem Int Ed 51:9592–9595
Zhang Y, Wang LZ, Zhang AQ, Song YH, Li XF, Feng H, Wu XB, Du PP (2010) Solid State Ionics 181:835–838
Jung Y, Kim S (2007) Electrochem Commun 9:249–254
Yamin H, Gorenshtein A, Penciner J, Sternberg Y, Peled E (1988) J Electrochem Soc 135:1045–1048
Li YJ, Zhan H, Liu SQ, Huang KL, Zhou YH (2010) J Power Sources 195:2945–2949
James RA, Yuriy VM, Neal W (2004) Solid State Ionics 175:243–245
Doron A, Elad P, Ran E, Gregory S, Scordilis K, John A (2009) J Electrochem Soc 156:A694–A702
Deng ZF, Zhang ZA, Lai YQ, Liu J, Li J, Liu YX (2013) J Electrochem Soc 160:A553–A558
Fu YZ, Manthiram A (2012) Chem Mater 24:3081–3087
Rao M, Song XY, Elton JC (2012) J Power Sources 205:474–478
Acknowledgments
The authors thank the financial support of the Strategic Emerging Industries Program of Shenzhen, China (JCYJ20120618164543322) and the Science and technology project of Hunan Province (2011FJ3151). We also thank the support of the Engineering Research Center of Advanced Battery Materials, the Ministry of Education, China.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, Q., Zhang, Z., Zhang, K. et al. Synthesis and electrochemical performance of TiO2–sulfur composite cathode materials for lithium–sulfur batteries. J Solid State Electrochem 17, 2959–2965 (2013). https://doi.org/10.1007/s10008-013-2203-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-013-2203-3