Abstract
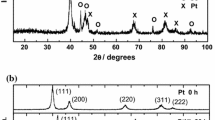
An array of Pd–W alloys was fabricated, and the electrocatalytic activity of the alloys for the oxygen reduction reaction (ORR) in acidic media was screened by scanning electrochemical microscopy. The Pd0.7W0.3 showed the highest activity for the ORR, close to that for Pd0.8Co0.2 and Pt. A Pd–W electrocatalyst loaded on carbon black was formed by the NaBH4-reduction method, exhibiting high activity and stability, suggesting that it is a good candidate for the proton exchange membrane fuel cell cathode.






Similar content being viewed by others
References
Reeve RW, Christensen PA, Hamnnett A, Haydock SA, Roy SC (1998) J Electrochem Soc 145:3463–3471
Reeve RW, Christensen PA, Dickinson AJ, Hamnett A, Scott K (2000) Electrochim Acta 45:4237–4250
Cong HN, Abbassi KE, Chartier PJ (2002) J Electrochem Soc 149:A525–A530
Alonso-Vante N, Malakhov IV, Nikitenko SG, Savinova ER, Kochubey DI (2002) Electrochim Acta 47:3807–3814
Fernández JL, Mano N, Heller A, Bard AJ (2004) Angew Chem Int Ed 43:6355–6357
Bron M, Hilgendorff M, Bogdanoff P (2002) J Appl Electrochem 32:211–216
Bron M, Radnik J, Fieber-Erdmann M, Bogdanoff P, Fiechter SJ (2002) J Electroanal Chem 535:113–119
Ralph TR, Hogarth MP (2002) Platinum Met Rev 46:3–14
Fernández JL, Bard AJ (2003) Anal Chem 75:2967–2974
Fernández JL, White JM, Sun Y, Tang W, Henkelman G, Bard AJ (2006) Langmuir 22:10426–10431
Fernández JL, Walsh DA, Bard AJ (2005) J Am Chem Soc 127:357–365
Fernández JL, Raghuveer V, Manthiram A, Bard AJ (2005) J Am Chem Soc 127:13100–13101
Walsh DA, Fernández JL, Bard AJ (2006) J Electrochem Soc 153:E99–E103
Sun YM, Alpuche-Aviles M, Bard AJ, Zhou JP, White JM (2009) J Nanosci Nanotechnol 9:1281–1286
Raghuveer V, Manthiram A, Bard AJ (2005) J Phys Chem B 109:22909–22912
Yu TH, Sha Y, Merinov BV, Goddard WA (2010) J Phys Chem C 114:11527–11533
Luigi P, Biago MB, Lorenzo A, Simona MT, Alessandro P (2008) Per Miner 77:63–73
Liu Z, Koh S, Yu C, Strasser PJ (2007) J Electrochem Soc 154:B1192–B1199
Lin C-L, Sánchez-Sánchez CM, Bard AJ (2008) Electrochem. Solid-State Lett 11:B136–B139
Acknowledgments
This work is supported by the National Science Foundation (CHE-1111518) and the International Cooperation Program for Excellent Lectures of 2008 by Shandong Provincial Education Department.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, W., Fan, FR.F. & Bard, A.J. The application of scanning electrochemical microscopy to the discovery of Pd–W electrocatalysts for the oxygen reduction reaction that demonstrate high activity, stability, and methanol tolerance. J Solid State Electrochem 16, 2563–2568 (2012). https://doi.org/10.1007/s10008-012-1775-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-012-1775-7