Abstract
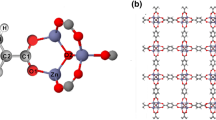
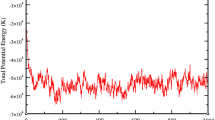
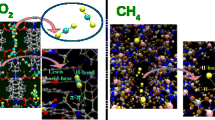
Molecular simulations were performed to predict CO2 adsorption in flexible metal-organic frameworks (MOFs). A generic force field was fitted to our experimental data to describe the non-bonded (electrostatic and van der Waals) interactions between CO2 molecules and the large pore (lp) and narrow pore (np) forms of the MIL-53(Al) framework. With the new validated force field, it is possible to predict CO2 uptake and enthalpy of adsorption at various applied external pressures that will modify the structure’s pore configuration and allow us to have more control over the adsorption/desorption process. A sensitivity analysis of MOF adsorption properties to the variation of the force field parameters was also intensively studied. It was shown that relatively small variations of the adsorbate gas model can improve the quality of the numerical predictions of the experimental data. However, the variations must be kept small enough to not modify the properties of the gas itself.









Similar content being viewed by others
References
Ramsahye NA, Maurin G, Bourrelly S et al (2007) On the breathing effect of a metal–organic framework upon CO2 adsorption: Monte Carlo compared to microcalorimetry experiments. Chem Commun 2007:3261–3263. doi:10.1039/b702986a
Salles F, Ghoufi A, Maurin G et al (2008) Molecular dynamics simulations of breathing MOFs: structural transformations of MIL-53(Cr) upon thermal activation and CO2 adsorption. Angew Chem Int Ed 47:8487–8491. doi:10.1002/anie.200803067
Schneemann A, Bon V, Schwedler I et al (2014) Flexible metal–organic frameworks. Chem Soc Rev 43:6062–6096. doi:10.1039/C4CS00101J
Serre C, Millange F, Thouvenot C et al (2002) Very large breathing effect in the first nanoporous chromium(III)-based solids: MIL-53 or Cr III (OH)·{O 2 C–C 6 H 4–CO 2}·{HO 2 C–C 6 H 4–CO 2 H} x·H 2 O y. J Am Chem Soc 124:13519–13526. doi:10.1021/ja0276974
Zhao X, Xiao B, Fletcher AJ et al (2004) Hysteretic adsorption and desorption of hydrogen by nanoporous metal-organic frameworks. Science 306:1012–1015. doi:10.1126/science.1101982
Bourrelly S, Llewellyn PL, Serre C et al (2005) Different adsorption behaviors of methane and carbon dioxide in the isotypic nanoporous metal terephthalates MIL-53 and MIL-47. J Am Chem Soc 127:13519–13521. doi:10.1021/ja054668v
García-Pérez E, Serra-Crespo P, Hamad S et al (2014) Molecular simulation of gas adsorption and diffusion in a breathing MOF using a rigid force field. Phys Chem Chem Phys 16:16060. doi:10.1039/C3CP55416C
Loiseau T, Serre C, Huguenard C et al (2004) A rationale for the large breathing of the porous aluminum terephthalate (MIL-53) upon hydration. Chem Eur J 10:1373–1382. doi:10.1002/chem.200305413
Serre C, Bourrelly S, Vimont A et al (2007) An explanation for the very large breathing effect of a metal–organic framework during CO2 adsorption. Adv Mater 19:2246–2251. doi:10.1002/adma.200602645
Dmol3, v. 8.0; Biovia Software, San Diego, 2014
Mulliken RS (1955) Electronic population analysis on LCAO[Single Bond]MO molecular wave functions. I. J Chem Phys 23:1833. doi:10.1063/1.1740588
Perdew JP, Yue W (1986) Accurate and simple density functional for the electronic exchange energy: generalized gradient approximation. Phys Rev B 33:8800
Harris JG, Yung KH (1995) Carbon dioxide’s liquid–vapor coexistence curve and critical properties as predicted by a simple molecular model. J Phys Chem 99:12021–12024. doi:10.1021/j100031a034
Maurin G, Llewellyn PL, Bell RG (2005) Adsorption mechanism of carbon dioxide in faujasites: grand canonical Monte Carlo simulations and microcalorimetry measurements. J Phys Chem B 109:16084–16091. doi:10.1021/jp052716s
Hamon L, Leclerc H, Ghoufi A et al (2011) Molecular insight into the adsorption of H 2 S in the flexible MIL-53(Cr) and rigid MIL-47(V) MOFs: infrared spectroscopy combined to molecular simulations. J Phys Chem C 115:2047–2056. doi:10.1021/jp1092724
Mayo SL, Olafson BD, Goddard WA (1990) DREIDING: a generic force field for molecular simulations. J Phys Chem 94:8897–8909
Rappé AK, Casewit CJ, Colwell KS et al (1992) UFF, a full periodic table force field for molecular mechanics and molecular dynamics simulations. J Am Chem Soc 114:100224
Ramsahye NA, Maurin G, Bourrelly S et al (2008) Probing the adsorption sites for CO2 in metal organic frameworks materials MIL-53 (Al, Cr) and MIL-47 (V) by density functional theory. J Phys Chem C 112:514–520. doi:10.1021/jp075782y
Sorption, v. 8.0; San Diego, Biovia Software, 2014
Akkermans RLC, Spenley NA, Robertson SH (2013) Monte Carlo methods in materials studio. Mol Simul 39:1153–1164. doi:10.1080/08927022.2013.843775
Ramsahye NA, Maurin G, Bourrelly S et al (2007) Charge distribution in metal organic framework materials: transferability to a preliminary molecular simulation study of the CO 2 adsorption in the MIL-53 (Al) system. Phys Chem Chem Phys 9:1059–1063. doi:10.1039/B613378A
Dubbeldam D, Calero S, Ellis DE, Snurr RQ (2016) RASPA: molecular simulation software for adsorption and diffusion in flexible nanoporous materials. Mol Simul 42:81–101. doi:10.1080/08927022.2015.1010082
Lemmon, EW, Huber ML, McLinden MO (2013) NIST standard reference database 23: reference fluid thermodynamic and transport properties-REFPROP, version 9.1, National Institute of Standards and Technology, Standard Reference Data Program, Gaithersburg
Desgranges C, Delhommelle J (2015) Many-body effects on the thermodynamics of fluids, mixtures, and nanoconfined fluids. J Chem Theory Comput 11:5401–5414. doi:10.1021/acs.jctc.5b00693
Liebrecht M, Cole MW (2012) Many-body effects in physical adsorption. J Low Temp Phys 169:316–323. doi:10.1007/s10909-012-0651-2
Acknowledgments
Supported by the Polish National Science Center (NCN, grant no. 2015/17/B/ST8/00099). Calculations were performed at the WCSS computer center of The Wroclaw University of Science and Technology using the Materials Studio Visualizer, Dmol3 and Sorption modules, grant no 33. E.D. would like to thank Ana Martín Calvo for numerous constructive discussions, and for her help with implementation of RASPA code.
Author information
Authors and Affiliations
Corresponding author
Additional information
This paper belongs to Topical Collection 7th Conference on Modeling & Design of Molecular Materials in Trzebnica (MDMM 2016)
Rights and permissions
About this article
Cite this article
Dundar, E., Chanut, N., Formalik, F. et al. Modeling of adsorption of CO2 in the deformed pores of MIL-53(Al). J Mol Model 23, 101 (2017). https://doi.org/10.1007/s00894-017-3281-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-017-3281-4