Abstract
Using density functional theory (DFT) and molecular dynamics (MD), we studied the interaction of a titanium atom with a half of a C60 fullerene (i.e., C30), formed from the corannulene structure with a pentagonal base. We considered atmospheric pressure and 300 K. We found that the most stable adsorption of the titanium atom on C30 occurs in the concave surface of the molecule. Afterward, we investigated the interaction of the system C30-titanium with carbon monoxide and carbon dioxide molecules, respectively. We found that each of these molecules is chemisorbed, with no dissociation. The value of the adsorption energy for the carbon monoxide molecule varies from −0.897 to −1.673 eV, and for the carbon dioxide molecule, it is between −1.065 and −1.274 eV. These values depend on the initial orientation of these molecules with respect to TiC30.

The TiC30 system chemisorbs CO or CO2ᅟwith no dissociation at atmospheric pressure and 300K
Similar content being viewed by others
Introduction
The development of new materials and technologies at the nanoscale, which could be used as hydrogen storage devices, sensors, catalysts, virus inhibitors, etc., has received much attention during recent years. In particular, carbon-based structures like graphene, carbon nanotubes, and fullerenes, have been widely studied. Although much work has been focused on graphene and carbon nanotubes due to their attractive mechanical and electronic properties, the C60 fullerene has different properties that recently have also raised great interest. These properties can change according to the several geometry variations considered. Their many promising applications span distinct fields like electronics, optical communications, biological systems, and medicine, in which their ability as a very good radical scavenger can be useful in drug delivery mechanisms [1–3]. Other studies have focused on their environmental potential uses as pollutant absorbents or sensors [4–6]. Currently, different techniques for the capture and storage of greenhouse gases like carbon monoxide and carbon dioxide are under study, and fullerenes emerge as an attractive option.
There are several theoretical and experimental studies of the interaction of the aforementioned pollutant molecules with carbon surfaces, or titanium-based surfaces [7–9]. Other investigations have focused on the chemisorption of a CO molecule on different catalysts made with transition metals. It has been shown that these metals can enhance the reaction speed, diffusion, and dissociation of carbon monoxide [10]. There have also been first-principles studies on the interaction between a CO2 molecule and the C60 fullerene, observing physisorption of the CO2 molecule [11]. Other experimental studies report the encasing of a CO molecule inside a C60 fullerene [12].
It is known that fragments of fullerenes based on the Corannulene aromatic bowl, are stable if certain conditions are satisfied, like the isolated pentagon rule. Different experimental and theoretical studies have found that these fragments share properties belonging to a complete fullerene, like the stabilization of neutral radicals. However, they also show particular properties not present in the fullerene. This fact increases their potential applications [13–16].
In this work, we studied the interaction of a titanium atom with half of a C60 fullerene (i.e., C30), originated from the corannulene structure with a pentagonal base. Then, we investigated the interaction of the system titanium-semifullerene with a CO molecule and with a CO2 molecule too. The simulations were performed at atmospheric pressure and 300 K. Understanding the mechanisms of adsorption of these molecules on this system is important in the development of further technological applications of semifullerenes.
Methods
Self-consistent field and molecular dynamics calculations were used in the study based on the density functional theory (DFT) [17], using the pseudopotential formalism. The Quantum Espresso code was used. This package considers a plane-wave expansion for the electronic wave functions, and periodic boundary conditions [18].
The Perdew-Burke-Ernzerhohof general gradient approximation (PBE-GGA) [19], was considered for the exchange and correlation functional. The norm conserving pseudo potentials are of the Martin-Troulliers type [20] for carbon and oxygen, and of the Vanderbilt type [21] for titanium. As valence electronic states we considered 2s2 and 2p2 for carbon, 2s2 and 2p4 for oxygen, and 3d2 and 4s2 for titanium.
The cut-off energy for the plane-wave set used was 80 Ry (≈1100 eV) and the convergence threshold for the energy was 1.2 × 10−5 eV. We considered 36 k-points within the Monkhorst-Pack scheme [22].
All calculations are non-relativistic, without polarized spin. The adsorption energies are calculated with E(ads) = E(system1 + system2) – E(system1) – E(system2). We employed the XCrySDen package for crystal structure visualization [23].
To validate our pseudopotentials we applied energy minimization, and we obtained, in the case of titanium, for the lattice parameter a = 2.863 Å and for c = 4.544 Å, the experimental values are 2.950 Å and 4.683 Å, respectively [24]. In the same manner, for carbon and oxygen, we optimized the CO2 molecule, obtaining for the C-O bond lengths 1.1614 and 1.1615 Å, with an angle between atoms of 179.99°. The experimental values are 1.163 Å and 180°, respectively [24].
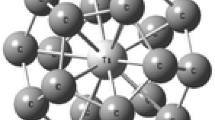
In this investigation, we consider one-half of a C60 fullerene. This semi-fullerene which we call C30, is based upon the corannulene structure. This molecule consists of 30 carbon atoms with a pentagonal base (Fig. 1). We optimized the C30, obtaining a length of 1.425 Å for the (5,6) single bond, and 1.360 Å for the (6,6) double bond (Fig. 1). These results are in good agreement with the experimental results (1.455 and 1.391 Å respectively) [25].
Afterward, we studied the interaction of a titanium atom with the C30. Then, we investigated the interactions, each at one time, of the system TiC30 with the pollutant molecules CO and CO2.
The system was confined in a cubic super cell with an edge size of 21.2 Å, to mimic vacuum conditions. For every interaction and for each initial alignment of the molecule under consideration, we allowed the complete system to follow an evolutionary process using molecular dynamics at 300 K, atmospheric pressure, and a time step of one femtosecond. We controlled the temperature in the MD calculations via velocity rescaling. The pressure in the Quantum Espresso code was controlled via a variable super cell size. The Parrinello-Raman barostat is used. The zero pressure corresponds to atmospheric pressure.
Results and discussion
Adsorption of a Ti atom on C30
From results in references [26, 27], we expect that the base of the C30 will be the most responsive region of the various sites, because of its curvature. In particular, in reference [27] the authors explored the sites of the more reactive electrons in the corannulene system (C20H10) with a pentagonal base, and in other molecules. Additionally, the electrostatic potential between the reactants is very important for the interaction. This electrostatic potential and the reactivity will determine the interaction. Clearly, the molecule C30 is different from C20H10, and the reactivity sites on the molecule depend on the dopants in it [26].
We studied two interaction cases. The first was with the titanium atom located near the concave face, initially at a distance of 1.52 Å from the plane defined by the border of the largest base of C30 (see Fig. 2a). The second was with the metal atom originally located near the convex face, at a distance of 3.11 Å, from the plane defined by the small base of C30 (see Fig. 3a). We found that, in both cases, the titanium atom is chemisorbed (see Figs. 2b and 3b). A Löwdin charge analysis [24] indicates that for the first case, there is a charge transfer from the titanium (−1.0917e) to all the carbon atoms in C30 (Fig. 2c). The transferred charge is distributed in a more or less regular way. In the second case, the charge transferred from the titanium atom (−0.8397e) is distributed mainly among the five carbon atoms located in the base of C30 (Fig. 3c). The adsorption energy for the first configuration is −6.762 eV while for the second case, we obtained −2.85 eV. Both final systems are stable. However, the first configuration, in which the titanium atom is adsorbed in the concave surface of C30, implies an adsorption stronger than that of the second case. We have chosen the most stable configuration of the system TiC30, to investigate the possible adsorption of the CO and CO2 molecules on it.
Adsorption of CO2 into the TiC30 system
The CO2 molecule is linear, and we placed it at two initial orientations with respect to C30. In the first case, the molecule is parallel to the plane defined by the base of C30. In the second, the molecule is perpendicular to the same plane. For the first case, the initial separation from the molecule to the plane defined by the largest base of C30, is 1.525 Å (Fig. 4a). In the second case, the introductory distance from the nearest oxygen atom of the molecule, to the same plane is also 1.525 Å (Fig. 5a).
We show molecular dynamics of the adsorption, onto TiC30 of the CO2 molecule. In the initial orientation, the carbon dioxide molecule is parallel to the plane defined by the base of C30. In the final configuration, the CO fraction of the CO2 molecule is tied to the titanium atom. The carbon dioxide molecule shows no dissociation. The simulations were performed at atmospheric pressure and 300K
We show the adsorption of the CO2 molecule onto TiC30. In the initial orientation, the carbon dioxide molecule is perpendicular to the plane defined by the base of C30. The final configuration shows no dissociation of the carbon dioxide molecule, with one oxygen atom tied to the titanium atom. The simulations were performed at atmospheric pressure and 300K
We found that in both cases, the CO2 molecule is chemisorbed with no dissociation.
In the final configuration for the first case, the fraction CO of the molecule is tied to the titanium atom of TiC30 (Fig. 4b). The adsorption energy is −1.065 eV. The CO2 molecule gains 0.3872 electrons from the TiC30 system. The number of electrons in the titanium atom decreases by an amount of 1.1011. For the second case, one of the oxygen atoms is tied to the titanium atom (Fig. 5b). The adsorption energy is −1.274 eV. The CO2 molecule transfers an amount of 0.0938 electrons to the TiC30 system, and the titanium atom loses 1.0963 electrons.
Adsorption of CO into the TiC30 system
For the CO molecule, we considered three initial orientations. In the first case, the molecule was parallel to the plane containing the base of TiC30 (see Fig. 6a). The second, is with the molecule perpendicular to the same above-mentioned plane and the oxygen atom facing the TiC30 (Fig. 7a), and the third is the same as in the second case, but with the carbon atom facing TiC30 (Fig. 8a). For all three cases, the molecule is chemisorbed with no dissociation, by the TiC30 system.
We show the adsorption of the CO molecule onto TiC30. In the initial orientation, the carbon dioxide molecule is parallel to the plane defined by the base of C30. The final configuration shows no dissociation of the carbon monoxide molecule, with the carbon atom tied to the titanium atom. The simulations were performed at atmospheric pressure and 300K
We show the adsorption of the CO molecule onto TiC30. In the initial orientation, the carbon dioxide molecule is perpendicular to the plane defined by the base of C30, with the oxygen atom facing the TiC30. The final configuration shows no dissociation of the carbon monoxide molecule, with the oxygen atom tied to the titanium atom. The simulations were performed at atmospheric pressure and 300K
We show the adsorption of the CO molecule onto TiC30. In the initial orientation, the carbon dioxide molecule is perpendicular to the plane defined by the base of C30, with the carbon atom facing the TiC30. The final configuration shows no dissociation of the carbon monoxide molecule, with the carbon atom tied to the titanium atom. The simulations were performed at atmospheric pressure and 300K
For the first case in the final configuration, the carbon atom of the CO molecule is tied to the titanium atom (Fig. 7b). The adsorption energy is −0.897 eV. The adsorbed molecule gains 0.1523 electrons from the TiC30 system. The titanium decreases its number of electrons by an amount of 1.0314. In the final configuration of the second case, the oxygen atom is tied to the titanium atom (Fig. 8b). The adsorption energy is −1.022 eV. The carbon monoxide molecule gains from the TiC30 system 0.0406 electrons. The titanium atom loses 1.0946 electrons. In the terminal configuration for the third case, the carbon atom is bound to the titanium atom, with an adsorption energy of −1.673 eV. The adsorbed molecule gains 0.1051 electrons. The titanium atom decreases its number of electron by 1.0025.
From the adsorption energies, the third orientation, where the carbon atom of CO was bound to the titanium atom was the most stable. The adsorption energy for this case is 86 % stronger than in the first orientation, and 64 % deeper than in the second case.
Conclusions
We found that the titanium atom can be chemisorbed on any (concave or convex) of the surfaces of C30. The case of the concave surface is the most stable, with an adsorption energy of −6.762 eV (which is 2.37 times the corresponding value for the second case).
We have chosen the most stable configuration of the system TiC30 for our investigation.
On the other hand, we found that the CO2 molecule is chemisorbed on the TiC30 system, without dissociation. The initial orientation of the molecule does not alter this result. However, the adsorption is most stable when the early orientation of the carbon dioxide molecule is perpendicular to the plane defined by the base of C30. In this case, the adsorption energy is −1.274 eV, which is 1.24 times the corresponding value for the other case.
For the case of the carbon monoxide molecule, we found that it is chemisorbed on TiC30 with no dissociation. The magnitude of the adsorption energy depends on the initial orientation of the molecule. The adsorption energy is the largest (−1.673 eV) when the molecule is perpendicular to the plane defined by the base of C30, with the carbon atom facing the titanium atom (Fig. 8a). The adsorption energy for this case is 86 % stronger than in the first orientation (Fig. 6a), and 64 % deeper than in the second case (Fig. 7a).
We performed our calculations considering atmospheric pressure and 300 K.
It is necessary to do the same kind of studies for more varied configurations in the future, and for different pollutant molecules. In this way, we could obtain a better understanding of the adsorption mechanisms in these graphene-based structures for environmental applications.
References
Bosi S, Da Ros T, Spalluto G, Prato M (2003) Fullerene derivatives: an attractive tool for biological applications. Eur J Med Chem 38:913–923
Georgakilas V, Perman JA, Tucek J, Zboril R (2015) Broad family of carbon nanoallotropes: classification, chemistry, and applications of fullerenes, carbon dots, nanotubes, graphene, nanodiamonds, and combined superstructures. Chem Rev 115:4744–4822
Mendes RG, Bachmatiuk A, Büchner B, Cuniberti G, Rümmeli MH (2013) Carbon nanostructures as multi-functional drug delivery platforms. J Mater Chem B 1:401–428
Scida K, Stege PW, Haby G, Messina GA, García CD (2011) Recent applications of carbon-based nanomaterials in analytical chemistry: critical review. Anal Chim Acta 691:6–17
Xie R-H, Bryant GW, Zhao J, Smith VH, Di Carlo A, Pecchia A (2003) Tailorable acceptor C60 − nBn and donor C60 − mNm pairs for molecular electronics. Phys Rev Lett 90:206602
Langa F, Nierengarten JSF (2010) Fullerene-rich nanostructures. In: Adv. Nanomater // Adv. Nanomater, pp 699–714
Kawasaki K, Sugita T, Ebisawa S (1967) Adsorption, surface reaction, and mutual displacement of CO, CO2 and O2 on titanium film. Surf Sci 7:502–506
Raupp GB, Dumesic JA (1985) Adsorption of carbon monoxide, carbon dioxide, hydrogen, and water on titania surfaces with different oxidation states. J Phys Chem 89:5240–5246
Mishra AK, Ramaprabhu S (2011) Carbon dioxide adsorption in graphene sheets. AIP Adv 1:032152
Zeinalipour-Yazdi CD, Cooksy AL, Efstathiou AM (2008) CO adsorption on transition metal clusters: trends from density functional theory. Surf Sci 602:1858–1862
Martínez-Alonso A, Tascón JMD, Bottani EJ (2001) Physical adsorption of Ar and CO 2 on C 60 fullerene. J Phys Chem B 105:135–139
Iwamatsu S, Stanisky CM, Cross RJ, Saunders M, Mizorogi N, Nagase S et al. (2006) Carbon monoxide inside an open-cage fullerene. Angew Chem Int Ed 45:5337–5340
Kroto HW (1987) The stability of the fullerenes Cn, with n = 24, 28, 32, 36, 50, 60 and 70. Nature 329:529–531
Scott LT (1996) Fragments of fullerenes: novel syntheses, structures and reactions. Pure Appl Chem 68:291–300
Chen MK, Hsin HJ, Wu T-C, Kang BY, Lee YW, Kuo MY et al. (2014) Highly curved bowl-shaped fragments of fullerenes: synthesis, structural analysis, and physical properties. Chem Eur J 20:598–608
Chen R, Lu R-Q, Shi K, Wu F, Fang H-X, Niu Z-X et al. (2015) Corannulene derivatives with low LUMO levels and dense convex–concave packing for n-channel organic field-effect transistors. Chem Commun 51:13768–13771
Parr RG, Yang W (1989) Density functional theory of atoms and molecules. Oxford University Press, Oxford
Giannozzi P, Baroni S, Bonini N, Calandra M, Car R, Cavazzoni C et al. (2009) QUANTUM ESPRESSO: a modular and open-source software project for quantum simulations of materials. J Phys Condens Matter 21:395502
Perdew JP, Burke K, Ernzerhof M (1996) Generalized gradient approximation made simple. Phys Rev Lett 77:3865–3868
Troullier N, Martıns JL (1991) Efficient pseudopotentials for planewave calculations. Phys Rev B 431:1993–2006
Vanderbilt D (1990) Soft self-consistent pseudopotentials in a generalized eigenvalue formalism. Phys Rev B 41:7892–7895
Monkhorst HJ, Pack JD (1976) Special points for Brillouin-zone integrations. Phys Rev B 13:5188–5192
Kokalj A (2003) Computer graphics and graphical user interfaces as tools in simulations of matter at the atomic scale. Comput Mater Sci 28:155–168
Lide DR (2013–2014) CRC handbook of chemistry and physics, 94th edn. CRC, Boca Raton, pp 9–16
David WIF, Ibberson RM, Matthewman JC, Prassides K, Dennis TJS, Hare JP et al. (1991) Crystal structure and bonding of ordered C60. Nature 353:147–149
Bulat FA, Burgess JS, Matis BR, Baldwin JW, Macaveiu L, Murray JS, Politzer P (2012) Hydrogenation and fluorination of graphene models: analysis via the average local ionization energy. J Phys Chem A 116:8644–8652
Murray JS, Shields ZP-I, Lane P, Macaveiu L, Bulat FA (2013) The average local ionization energy as a tool for identifyinbrsg reactive sites on defect-containing model graphene systems. J Mol Model 2825–2833
Acknowledgments
The authors wish to thank Dirección General de Asuntos del Personal Académico de la Universidad Nacional Autónoma de México for the partial financial support by Grant IN-106514 and UNAM super-computing center for the technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Canales, M., Ramírez-de-Arellano, J.M. & Magana, L.F. Interaction of a Ti-doped semi-fullerene (TiC30) with molecules of CO and CO2 . J Mol Model 22, 223 (2016). https://doi.org/10.1007/s00894-016-3086-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-016-3086-x