Abstract
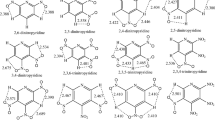
Stabilities of pyridylpentazoles, pyridazylpentazoles, triazinylpentazoles, tetrazinylpentazoles, and pentazinylpentazole were studied using density functional theory to assess their potentials as the source of pentazole anion (N5 −) for replacement of phenylpentazole (PhN 5 ). Replacing the aryl group of PhN 5 by six-member heterocycle weakens pentazole ring. Compared to PhN 5 , title molecules have longer N-N bonds and lower activation energy (E a,1) needed for the N5 ring breaking. E a,1 decreases with the increasing number of nitrogen atoms of heterocycle. The ortho nitrogen of heterocycle most obviously lowers the stability of pentazole. The central C-N bond dissociation energies (BDEs) of title molecules are lower than that of PhN 5 . For the molecule with 0~1 ortho-nitrogen, H rearrangement happens during the central C-N bond breaking. The energy (E a,2) required for H rearrangement is considerably smaller than the corresponding BDE. ΔE a,2 (E a,2(PhN5) - E a,2 = 7.5~35.7 kJ mol-1) is larger than ΔE a,1 (E a,2(PhN5) - E a,2 = 4.6~15.5 kJ mol-1), while ΔE a,2/E a,2(PhN5) (2~9.5 %) is smaller than ΔE a,1/E a,1(PhN5) ( 4.4~15.0 %). The larger ΔE a,1/E a,1(PhN5) suggests that title molecules can not be the better N5 − than PhN 5 .



Similar content being viewed by others
References
Christe KO, Wilson WW, Sheehy JA, Boatz JA (1999) N5 +: a novel homoleptic polynitrogen ion as a high energy density material. Angew Chem Int Ed 38(13–14):2004–2009
Gagliardi L, Pyykkö P (2002) η5-N5 −-Metal-η7-N7 3−: a new class of compounds. J Phys Chem A 106(18):4690–4694
Nguyen MT (2006) Polynitrogen compounds: 1. Structure and stability of N4 and N5 systems. Coord Chem Rev 244(1–2):93–113
Belau L, Haas Y, Zilberg S (2004) Formation of the cyclo-pentazolate N5 − anion by high-energy dissociation of phenylpentazole anions. J Phys Chem A 108(52):11715–11720
Carlqvist P, Östmark H, Brinck T (2004) The stability of arylpentazoles. J Phys Chem A 108:7463–7467
Strout DL (2004) Isomer stability of N24, N30, and N36 cages: cylindrical versus spherical structure. J Phys Chem A 108(13):2555–2558
Wang L, Mezey PG (2005) Predicted high-energy molecules: helical all-nitrogen and helical nitrogen-rich ring clusters. J Phys Chem A 109(14):2341–2343
Wua HS, Xua XH, Jiao H (2005) Structure and stability of perazido substituted azacycloalkanes, Nn(N3)n. Chem Phys Lett 412(4–6):299–302
Colvin KD, Strout DL (2005) Stability of nitrogen-oxygen cages N12O2, N14O2, N14O3, and N16O4. J Phys Chem A 109(35):8011–8015
Vij A, Wilson WW, Vij V, Tham FS, Sheehy JA, Christe KO (2001) Polynitrogen chemistry. Synthesis, characterization, and crystal structure of surprisingly stable fluoroantimonate salts of N5 +. J Am Chem Soc 123(26):6308–6313
Cacace F, Petris G, Troiani A (2002) Experimental detection of tetranitrogen. Science 295:480–481
Vij A, Pavlovich JG, Wilson WW, Vij V, Christe KO (2002) Experimental detection of the pentaazacyclopentadienide (pentazolate) anion, cyclo-N5 −. Angew Chem 114(16):3177–3180
Östmark H, Wallin S, Brinck T, Carlqvist P, Claridge R, Hedlund E, Yudina L (2003) Detection of pentazolate anion (cyclo-N5 −) from laser ionization and decomposition of solid p-dimethylaminophenylpentazole. Chem Phys Lett 379(5):539–546
Fau S, Wilson KJ, Bartlett RJ (2002) On the stability of N5 + N5 −. J Phys Chem A 106(18):4639–4644
Lein M, Frunzke J, Timoshkin A, Frenking G (2001) Iron bispentazole Fe (η5-N5)2, a theoretically predicted high‐energy compound: structure, bonding analysis, metal–ligand bond strength and a comparison with the isoelectronic ferrocene. Chem Eur J 7(19):4155–4163
Tang L, Guo H, Peng J, Ning P, Li K, Li J, Gu J, Li Q (2014) Structure and bonding of novel paddle-wheel diiridium polynitrogen compounds: a stronger iridiumeiridium bonding by density functional theory. J Organomet Chem 769:94–99
Zhang X, Yang J, Lu M, Gong X (2014) Theoretical studies on stability and pyrolysis mechanism of salts formed by N5 − and metallic cations Na+, Fe2+ and Ni2+. Struct Chem 1–8
Zhao JF, Li N, Li QS (2003) A kinetic stability study of MN5 (M = Li, Na, K, and Rb). Theor Chem Accounts 110(1):10–18
Zhang XH, Li S, Li QS (2006) Characterizations of novel binuclear alkaline-earth metal compounds: M2(ηn-N5)2 (M = Be and Mg, n = 1, 2; M = Ca, n = 2, 5). J Theor Comput Chem 05:475–487
Tang LH, Guo HB, Li QS, Peng JH, Gu JJ, Xiao LB (2014) Characterizations of novel binuclear transition metal polynitrogen compounds: M2(N5)4 (M = Co, Rh and Ir). Adv Mater Res 924:233–252
Zhang X, Yang J, Lu M, Gong X (2015) Structure, stability and intramolecular interaction of M (N5)2 (M = Mg, Ca, Sr and Ba): a theoretical study. RSC Adv 5(28):21823–21830
Cui J, Zhang Y, Zhao F, Yang J, Shen G, Xua Y (2009) HB(N5)3M (M = Li, Na, K, and Rb): a new kind of pentazolides as HEDMs. Prog Nat Sci 19:41–45
Benin V, Kaszynski P, Radziszewski G (2002) Arylpentazoles revisited: experimental and theoretical studies of 4-hydroxyphenylpentazole and 4-oxophenylpentazole anion. J Org Chem 67(4):1354–1358
Butler RN, Hanniffy JM, Stephens JC, Burke LA (2008) A ceric ammonium nitrate N-dearylation of N-p-anisylazoles applied to pyrazole, triazole, tetrazole, and pentazole rings: release of parent azoles. generation of unstable pentazole, HN5/N5 −, in solution. J Org Chem 73(4):1354–1364
Zhang XL, Yang JQ, Lu M, Gong XD (2014) Theoretical studies on the stability of phenylpentazole and its substituted derivatives of–OH, −OCH3, −OC2H5 and –N(CH3)2. RSC Adv 4(99):56095–56101
Zhang X, Yang J, Lu M, Gong X (2015) Pyridylpentazole and its derivatives: a new source of N5 −? RSC Adv 5(35):27699–27705
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2004). Gaussian 03, revision C02. Gaussian Inc, Wallingford
Simón L, Goodman JM (2011) How reliable are DFT transition structures? Comparison of GGA, hybrid-meta-GGA and meta-GGA functionals. Org Biomol Chem 9(3):689–700
Wu Q, Zhu W, Xiao H (2014) A new design strategy for high-energy low-sensitivity explosives: combining oxygen balance equal to zero, a combination of nitro and amino groups, and N-oxide in one molecule of 1-amino-5-nitrotetrazole-3 N-oxide. J Mater Chem A 2(32):13006–13015
Yu T, Zheng J, Truhlar DG (2011) Multi-structural variational transition state theory. Kinetics of the 1, 4-hydrogen shift isomerization of the pentyl radical with torsional anharmonicity. Chem Sci 2(11):2199–2213
Franzen S, Skalski B, Bartolotti L, Delley B (2014) The coupling of tautomerization to hydration in the transition state on the pyrimidine photohydration reaction path. Phys Chem Chem Phys
Benson SW (1976) Thermochemical kinetics: methods for the estimation of thermochemical data and rate parameters. Wiley, New York
Yao XQ, Hou XJ, Wu GS, Xu YY, Xiang HW, Jiao H, Li YW (2002) Estimation of CC bond dissociation enthalpies of large aromatic hydrocarbon compounds using DFT methods. J Phys Chem A 106(31):7184–7189
Shao J, Cheng X, Yang X (2005) Density functional calculations of bond dissociation energies for removal of the nitrogen dioxide moiety in some nitroaromatic molecules. J Mol Struct (THEOCHEM) 755(1):127–130
Fan XW, Ju XH, Xia QY, Xiao HM (2008) Strain energies of cubane derivatives with different substituent groups. J Hazard Mater 151(1):255–260
Kamijo S, Jin T, Huo Z, Gyoung YS, Shim JG, Yamamoto Y (2003) Tetrazole synthesis via the palladium-catalyzed three component coupling reaction. Mol Divers 6(3–4):181–192
Geiger U, Elyashiv A, Fraenkel R, Zilberg S, Haas Y (2013) The Raman spectrum of dimethylaminophenyl pentazole (DMAPP). Chem Phys Lett 556:127–131
Portius P, Davis M, Campbell R, Hartl F, Zeng Q, Meijer AJ, Towrie M (2013) Dinitrogen release from arylpentazole: a picosecond time-resolved infrared, spectroelectrochemical, and DFT computational study. J Phys Chem A 117(48):12759–12769
Geiger U, Haas Y, Grinstein D (2014) The photochemistry of an aryl pentazole in liquid solutions: the anionic 4-oxidophenylpentazole (OPP). J Photochem Photobiol A Chem 277:53–61
Li QS, Hu XG, Xu WG (1998) Structure and stability of N 7 cluster. Chem Phys Lett 287(1):94–99
Canneaux S, Bohr F, Hénon E (2014) KiSThelP: a program to predict thermodynamic properties and rate constants from quantum chemistry results. J Comput Chem 35:82–93
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
(DOC 1996 kb)
Rights and permissions
About this article
Cite this article
Zhang, X., Gong, X. Theoretical investigations on stability of pyridylpentazoles, pyridazylpentazoles, triazinylpentazoles, tetrazinylpentazoles, and pentazinylpentazole searching for a replacement of phenylpentazole as N5 − source. J Mol Model 21, 318 (2015). https://doi.org/10.1007/s00894-015-2867-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-015-2867-y