Abstract
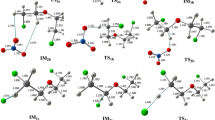
Ab initio calculations were performed to study the quantum chemistry reactions mechanisms among Hg0, elemental halogen and O3. The geometry of reactions, transition states (TS), intermediates (M) and products were optimized using the MP2 method at the SDD basis function level for Hg, and using 6-311++G (3df, 3pd) for other species. Molecular energies were calculated at QCISD (T) level with zero point energy. Activation energies were calculated along with pre-exponential factors . The reaction rate constants within 298–1800 K were calculated according to transition state theory (TST). The influences of O3 on the reaction of Hg0 with halogen are discussed. Hg0 can be oxidized to Hg1+ by halogen and O3, and halogen and O3 can be arranged in decreasing order as: Br2 > BrO > O3 > Br > Cl, BrCl > HBr > HCl, Br2 > Cl2 according to reaction rate constants. When O3 is presented, Br2, HBr, BrCl, Cl2 and HCl react with O3 and are initially converted to BrO and ClO. O3 is unfavorable for oxidation of Hg0 by Br2. The mixture of HBr and O3 has better oxidizing Hg0 performance than HBr and O3. Cl is less effective than Br for oxidation of Hg0.












Similar content being viewed by others
References
Zhang L, Wong MH (2007) Environmental mercury contamination in China: sources and impacts. Environ Int 33(1):108–121
Yudovich YE, Ketris MP (2005) Mercury in coal: review part 2. Coal use and environmental problem. Int J Coal Geol 62(3):135–165
Yan NQ, Liu SH, Chang SG, Miller C (2005) Method for the study of gaseous oxidants for the oxidation of mercury gas. Ind Eng Chem Res 44(15):5567–5574
Senior CL, Sarofim AF, Zeng T, Helble JJ, Mamani-Paco R (2000) Gas-phase transformations of mercury in coal-fired power plants. Fuel Process Technol 63:197–213
Eswaran S, Stenger HG (2008) Effect of halogens on mercury conversion in SCR catalysts. Fuel Process Technol 89:1153–1159
Rutter AP, Shakya KM, Lehr R, Schauer JJ, Griffin RJ (2012) Oxidation of gaseous elemental mercury in the presence of secondary organic aerosols. Atmos Environ 59:86–92
Lee S-S, Lee J-Y, Keener TC (2009) Mercury oxidation and adsorption characteristics of chemically promoted activated carbon sorbents. Fuel Process Technol 90:1314–1318
Agarwal H, Stenger HG (2007) Development of a predictive kinetic model for homogeneous Hg oxidation data. Math Comput Model 45:109–125
Tao Y, Zhuo Y, Zhang L, Chen C, Xu X (2010) Impact of flue gas species and temperature on mercury oxidation. Tsinghua Sci Technol 15(4):418–425
Goodsite ME, Plane JMC, Skov H (2004) A theoretical study of the oxidation of Hg0 to HgBr2 in the troposphere. Environ Sci Technol 38:1772–1776
Balabanov NB, Peterson KA (2003) Mercury and reactive halogens: the thermochemistry of Hg + {Cl2, Br2, BrCl, ClO, and BrO}. J Phys Chem A 107:7465–7470
Tossell JA (2003) Calculation of the energetics for oxidation of gas-phase elemental Hg by Br and BrO. J Phys Chem A 107:7804–7808
Niksa S, Naik CV, Berry MS, Monroe L (2009) Interpreting enhanced Hg oxidation with Br addition at plant miller. Fuel Process Technol 90:1372–1377
Calvert JG, Lindberg SE (2005) Mechanisms of mercury removal by O3 and OH in the atmosphere. Atmos Environ 39:3355–3367
Ye Z, Zygarlicke CJ, Galbreath KC, Thompson JS, Holmes MJ, Pavlish JH (2004) Kinetic transformation of mercury in coal combustion flue gas in a bench-scale entrained- flow reactor. Fuel Process Technol 85:463–472
Hongliang G, Jinsong Z, Zhongyang L, Kefa C (2007) Experimental study on hg vapor adsorption of modified activated carbons in simulated flue gas. J Power Eng 27(8):26–30
Liu Jing Q, Wenqi ZC (2013) Theoretical studies of mercury-Br species adsorption mechanism on carbonaceous surface. Proc Combust Inst 34:2811–2819
Sliger RN, Kramlich JC, Marinov NM (2000) Towards the development of a chemical kinetic model for the homogeneous oxidation of mercury by chlorine species. Fuel Process Technol 65–66:423–438
Xu M, Qiao Y, Zheng C, Li L, Liu J (2003) Modeling of homogeneous mercury speciation using detailed chemical kinetics. Combust Flame 132:218
Castro L, Dommergue A, Ferrari C, Maron L (2009) A DFT study of the reaction of O3 with Hg0 or Br. Atmos Environ 43:5708–5711
Taylor PH, Mallipeddi R, Yamada T (2005) LP/LIF study of the formation and consumption of mercury (I) chloride: kinetics of mercury chlorination. Chemosphere 61:685–692
Shon Z-H, Kim K-H, Kim M-Y, Lee M (2005) Modeling study of reactive gaseous mercury in the urban air. Atmos Environ 39:749–761
Subir M, Ariya PA, Dastoor AP (2011) A review of uncertainties in atmospheric modeling of mercury chemistry I. Uncertainties in existing kinetic parameters e fundamental limitations and the importance of heterogeneous chemistry. Atmos Environ 45:5664–5676
Xu M, Qiao Y, Liu J, Zheng C (2008) Kinetic calculation and modeling of trace element reactions during combustion. Powder Technol 180(1):157–163
Krishnakumar B, Helble JJ (2012) Determination of transition state theory rate constants to describe mercury oxidation in combustion systems mediated by Cl, Cl 2, HCl and HOCl. Fuel Process Technol 94(1):1–9
Zheng C, Liu J, Liu Z, Xu M, Liu Y (2005) Kinetic mechanism studies on reaction of mercury and oxidizing species in coal combustion. Fuel 84:1215–1220
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gao, Z., Lv, S., Yang, W. et al. Quantum chemistry investigation on the reaction mechanism of the elemental mercury, chlorine, bromine and ozone system. J Mol Model 21, 160 (2015). https://doi.org/10.1007/s00894-015-2707-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-015-2707-0