Abstract
We have investigated computationally the effects of π-conjugation extension on naphtha[2,1-b:6,5-b’] difuran (DPNDF); where we increase the number of fused NDF (central core) and furan rings in the parent molecule. The molecular structures of all analogues have been optimized at the ground (S0) and first excited (S1) states using density functional theory (DFT) and time-dependent density functional theory (TD-DFT), respectively. Then highest occupied molecular orbitals (HOMOs), the lowest unoccupied molecular orbitals (LUMOs), photophysical properties, adiabatic/vertical electron affinities (EAa)/(EAv), adiabatic/vertical ionization potentials (IPa)/(IPv), and hole/electron reorganization energies λh/λe have been investigated. The effect of NDF and furan rings on structural and electro-optical properties has also been studied. Our calculated reorganization energies of 1a, 1b, and 2c reveal them, materials with balanced hole/electron charge transport, whereas 2a and 2b are good hole-transport materials. By increasing the number of furan rings; the photostability was augmented in 2a, 2b, and 2c.

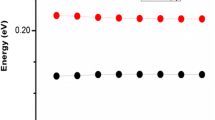
Computed emission spectra, at the TD-B3LYP/6-31G** level of theory







Similar content being viewed by others
References
Reshak AH, Stys D, Auluck S, Kityk IV (2010) Density functional calculations of the electronic structure of 3-phenylamino-4-phenyl-1,2,4-triazole-5-thione. Phys Chem Phys 12(12):2975–2980
Reshak AH, Stys D, Auluck S, Kityk IV (2009) Ab initio calculation of the electronic band structure, density of states and optical properties of α-2-methyl-1-nitroisothiourea. J Phys Chem B 113(38):12648–12654
Reshak AH, Stys D, Auluck S, Kityk IV (2010) Linear and nonlinear optical susceptibilities of 3-phenylamino-4-phenyl-1,2,4-triazole-5-thione. J Phys Chem B 114(5):1815–1821
Pokladko M, Gondek E, Sanetra J, Nizioł J, Danel A, Kityk IV, Reshak AH (2009) Spectral emission properties of 4-aryloxy-3-methyl-1-phenyl-1H-pyrazolo[3,4-b]quinolines. Spectrochim Acta A Mol Biomol Spectrosc 73(2):281–285
Fuks-Janczarek I, Reshak AH, Kuźnik W, Kityk IV, Gabański R, Lapkowski M, Motyka R, Suwiński J (2009) UV–vis absorption spectra of 1,4-dialkoxy-2,5-bis[2-(thien-2-yl)ethenyl]benzenes. Spectrochim Acta A Mol Biomol Spectrosc 72(2):394–398
Wojciechowski A, Ozga K, Reshak AH, Miedzinski R, Kityk IV, Berdowski J, Tylczyński Z (2010) Photoinduced effects in l-alanine crystals. Mater Lett 64(18):1957–1959
Matsumi N, Naka K, Chujo Y (1998) Extension of π-conjugation length via the vacant p-orbital of the boron atom. Synthesis of novel electron deficient π-conjugated systems by hydroboration polymerization and their blue light emission. J Am Chem Soc 120(20):5112–5113
Morley JO, Docherty VJ, Pugh D (1987) Non-linear optical properties of organic molecules. Part 2 effect of conjugation length and molecular volume on the calculated hyperpolarisabilities of polyphenyls and polyenes. J Chem Soc Perkin Trans 2(9):1351–1355
Monkman AP, Burrows HD, Hamblett I, Navarathnam S, Svensson M, Andersson MR (2001) The effect of conjugation length on triplet energies, electron delocalization and electron–electron correlation in soluble polythiophenes. J Chem Phys 115(19):9046–9049
Meier H, Stalmach U, Kolshorn H (1997) Effective conjugation length and UV/vis spectra of oligomers. Acta Polym 48(9):379–384
Moussallem C, Gohier F, Mallet C, Allain M, Frère P (2012) Extended benzodifuran–furan derivatives as example of π-conjugated materials obtained from sustainable approach. Tetrahedron 68(41):8617–8621
Kim R, Amegadze PSK, Kang I, Yun H-J, Noh Y-Y, Kwon S-K, Kim Y-H (2013) High-mobility Air-stable naphthalene diimide-based copolymer containing extended π-conjugation for n-channel organic field effect transistors. Adv Funct Mater 23(46):5719–5727
Oldham WJ, Miao Y-J, Lachicotte RJ, Bazan GC (1998) Stilbenoid dimers: effect of conjugation length and relative chromophore orientation. J Am Chem Soc 120(2):419–420
Reshak AH, Kamarudin H, Auluck S (2013) Electronic structure, density of electronic states, and the chemical bonding properties of 2,4-dihydroxyl hydrazone crystals (C13H11N3O4). J Mater Sci 48(10):3805–3811
Reshak AH, Kamarudin H, Kityk IV, Auluck S (2013) Electronic structure, charge density, and chemical bonding properties of C11H8N2O o-methoxydicyanovinylbenzene (DIVA) single crystal. J Mater Sci 48(15):5157–5162
Reshak AH, Kamarudin H, Auluck S (2012) Acentric nonlinear optical 2,4-dihydroxyl hydrazone isomorphic crystals with large linear nonlinear optical susceptibilities and hyperpolarizability. J Phys Chem B 116(15):4677–4683
Reshak AH, Auluck S, Stys D, Kityk IV, Kamarudin H, Berdowski J, Tylczynski Z (2011) Dispersion of linear and non-linear optical susceptibilities for amino acid 2-aminopropanoic CH3CH(NH2)COOH single crystals: experimental and theoretical investigations. J Mater Chem 21(43):17219–17228
Reshak AH, Stys D, Auluck S, Kityk IV, Kamarudin H (2011) Structural properties and bonding nature of 3-methyl-4-phenyl-5-(2-pyridyl)-1,2,4-triazole single crystal. Mater Chem Phys 130(1–2):458–465
Irfan A (2014) First principle investigations to enhance the charge transfer properties by bridge elongation. J Theor Comput Chem 13(02):1450013
Muhammad S, Irfan A, Shkir M, Chaudhry AR, Kalam A, AlFaify S, Al-Sehemi AG, Al-Salami AE, Yahia IS, Xu HL, Su ZM (2014) How does hybrid bridging core modification robust the nonlinear optical properties in donor-π-acceptor configuration? a case study of dinitrophenol derivatives. J Comput Chem. doi:10.1002/jcc.23777
Irfan A, Al-Sehemi AG, Al-Assiri MS (2013) Modeling of multifunctional donor-bridge-acceptor 4, 6-di (thiophen-2-yl) pyrimidine derivatives: A first principles study. J Mol Graphics Modell 44:168–176
Irfan A, Jin R, Al-Sehemi AG, Asiri AM (2013) Quantum chemical study of the donor-bridge-acceptor triphenylamine based sensitizers. Spectrochim Acta A Mol Biomol Spectrosc 110:60–66
Irfan A (2014) Influence of the substitution on the electronic properties of perylene-3, 4: 9, 10-bis (dicarboximides): density functional theory study. Bull Chem Soc Ethiop 28(1):101–110
Irfan A, Ijaz F, Al-Sehemi A, Asiri A (2012) Quantum chemical approach toward rational designing of highly efficient oxadiazole based oligomers used in organic field effect transistors. J Comput Electron 11(4):374–384
Katz EH (1997) Organic molecular solids as thin film transistor semiconductors. J Mater Chem 7(3):369–376
Horowitz G, Hajlaoui ME (2000) Mobility in polycrystalline oligothiophene field-effect transistors dependent on grain size. Adv Mater 12(14):1046–1050
Newman CR, Frisbie CD, da Silva Filho DA, Brédas J-L, Ewbank PC, Mann KR (2004) Introduction to organic thin film transistors and design of n-channel organic semiconductors. Chem Mater 16(23):4436–4451
Nakano M, Shinamura S, Houchin Y, Osaka I, Miyazaki E, Takimiya K (2012) Angular-shaped naphthodifurans, naphtho[1,2-b;5,6-b’]- and naphtho[2,1-b;6,5-b’]-difuran: are they isoelectronic with chrysene? Chem Commun 48(45):5671–5673
Tang CW, VanSlyke SA (1987) Organic electroluminescent diodes. Appl Phys Lett 51(12):913–915
Ho PKH, Kim J-S, Burroughes JH, Becker H, Li SFY, Brown TM, Cacialli F, Friend RH (2000) Molecular-scale interface engineering for polymer light-emitting diodes. Nature 404(6777):481–484
Al-Sehemi AG, Irfan A, Asiri AM (2014) Red and yellow color aspects of compound 3-dicyclopropylmethylene-5-dicyanomethylene-4-diphenylmethylenetetrahydrofuran-2-one chromism effect. Chin Chem Lett 25(4):609–612
Padinger F, Rittberger RS, Sariciftci NS (2003) Effects of postproduction treatment on plastic solar cells. Adv Funct Mater 13(1):85–88
Irfan A (2013) Quantum chemical investigations of electron injection in triphenylamine-dye sensitized TiO2 used in dye sensitized solar cells. Mater Chem Phys 142(1):238–247
Wrackmeyer MS, Hein M, Petrich A, Meiss J, Hummert M, Riede MK, Leo K (2011) Dicyanovinyl substituted oligothiophenes thermal stability, mobility measurements, and performance in photovoltaic devices. Sol Energy Mater Sol Cells 95(12):3171–3175
Irfan A, Nadeem M, Athar M, Kanwal F, Zhang J (2011) Electronic, optical and charge transfer properties of α, α’-bis(dithieno[3,2-b:2’,3’- d]thiophene) (BDT) and its heteroatom-substituted analogues. Comput Theor Chem 968(1–3):8–11
Irfan A, Al-Sehemi AG, Muhammad S, Zhang J (2011) Packing effect on the transfer integrals and mobility in α, α’-bis(dithieno[3,2-b:2’,3’-d]thiophene) (BDT) and its heteroatom-substituted analogues. Aust J Chem 64(12):1587–1592
Das S, Senanayak SP, Bedi A, Narayan KS, Zade SS (2011) Synthesis and charge carrier mobility of a solution-processable conjugated copolymer based on cyclopenta[c]thiophene. Polymer 52(25):5780–5787
Letizia JA, Cronin S, Ortiz RP, Facchetti A, Ratner MA, Marks TJ (2010) Phenacyl–thiophene and quinone semiconductors designed for solution processability and air-stability in high mobility n-channel field-effect transistors. Chem Eur J 16(6):1911–1928
Buonocore F, Matteo A (2009) Energetic of molecular interface at metal-organic heterojunction: the case of thiophenethiolate chemisorbed on Au(111). Theor Chem Acc 124(3–4):217–223
Wu Q-X, Geng Y, Liao Y, Tang X-D, Yang G-C, Su Z-M (2012) Theoretical studies of the effect of electron-withdrawing dicyanovinyl group on the electronic and charge-transport properties of fluorene-thiophene oligomers. Theor Chem Acc 131(3):1–9
Unni KNN, Dabos-Seignon S, Nunzi J-M (2006) Influence of the polymer dielectric characteristics on the performance of a quaterthiophene organic field-effect transistor. J Mater Sci 41(2):317–322
Pingel P, Zen A, Neher D, Lieberwirth I, Wegner G, Allard S, Scherf U (2009) Unexpectedly high field-effect mobility of a soluble, low molecular weight oligoquaterthiophene fraction with low polydispersity. Appl Phys A 95(1):67–72
Koezuka H, Tsumura A, Ando T (1987) Field-effect transistor with polythiophene thin film. Synth Met 18(1–3):699–704
Mikhailov IA, Belfield KD, Masunov AE (2009) DFT-based methods in the design of Two-photon operated molecular switches. J Phys Chem A 113(25):7080–7089
Irfan A, Al-Sehemi AG, Muhammad S (2014) Push-pull effect on the charge transport properties in anthra [2, 3-b] thiophene derivatives used as dye-sensitized and hetero-junction solar cell materials. Synth Met 190:27–33
Irfan A, Al-Sehemi AG, Al-Assiri MS (2014) The effect of donors–acceptors on the charge transfer properties and tuning of emitting color for thiophene, pyrimidine and oligoacene based compounds. J Fluorine Chem 157:52–57
Irfan A, Chaudhry AR, Al-Sehemi AG, Al-Asiri MS, Muhammad S, Kalam A (2014) Investigating the effect of acene-fusion and trifluoroacetyl substitution on the electronic and charge transport properties by density functional theory. J Saudi Chem Soc. doi:10.1016/j.jscs.2014.09.009 (0)
Irfan A (2014) Highly efficient renewable energy materials benzo[2,3-b]thiophene derivatives: electronic and charge transfer properties study Optik. Int J Light Electron Optics 125(17):4825–4830
Irfan A, Al-Sehemi AG, Al-Assiri MS (2014) Push–pull effect on the electronic, optical and charge transport properties of the benzo[2,3-b]thiophene derivatives as efficient multifunctional materials. Comput Theor Chem 1031:76–82
Irfan A (2014) Modeling of efficient charge transfer materials of 4,6-di(thiophen-2-yl)pyrimidine derivatives quantum chemical investigations computational. Mater Sci 81:488–492
Miyata Y, Nishinaga T, Komatsu K (2005) Synthesis and structural, electronic, and optical properties of oligo(thienylfuran)s in comparison with oligothiophenes and oligofurans. J Org Chem 70(4):1147–1153
Miyata Y, Terayama M, Minari T, Nishinaga T, Nemoto T, Isoda S, Komatsu K (2007) Synthesis of oligo(thienylfuran)s with thiophene rings at both ends and their structural, electronic, and field-effect properties. Chem–Asian J 2(12):1492–1504
Gidron O, Dadvand A, Sheynin Y, Bendikov M, Perepichka DF (2011) Towards “green” electronic materials. α-oligofurans as semiconductors. Chem Commun 47(7):1976–1978
Wu C-C, Hung W-Y, Liu T-L, Zhang L-Z, Luh T-Y (2003) Hole-transport properties of a furan-containing oligoaryl. J Appl Phys 93(9):5465–5471
Kadac K, Bosiak MJ, Nowaczyk J (2012) Synthesis and AC impedance studies of 2,6-distyrylbenzofuro[5,6-b]furan based new organic semiconductor. Synth Met 162(21–22):1981–1986
Nakano M, Niimi K, Miyazaki E, Osaka I, Takimiya K (2012) Isomerically pure anthra[2,3-b:6,7-b’]-difuran (anti-ADF), −dithiophene (anti-ADT), and -diselenophene (anti-ADS): selective synthesis electronic structures, and application to organic field-effect transistors. J Org Chem 77(18):8099–8111
Niimi K, Mori H, Miyazaki E, Osaka I, Kakizoe H, Takimiya K, Adachi C (2012) [2,2[prime or minute]]Bi[naphtho[2,3-b]furanyl]: a versatile organic semiconductor with a furan-furan junction. Chem Commun 48(47):5892–5894
Watanabe M, Su W-T, Chang YJ, Chao T-H, Wen Y-S, Chow TJ (2013) Solution-processed optoelectronic properties of functionalized anthradifuran. Chem–Asian J 8(1):60–64
Chen H, Delaunay W, Li J, Wang Z, Bouit P-A, Tondelier D, Geffroy B, Mathey F, Duan Z, Réau R, Hissler M (2013) Benzofuran-fused phosphole: synthesis electronic, and electroluminescence properties. Org Lett 15(2):330–333
Mitsudo K, Harada J, Tanaka Y, Mandai H, Nishioka C, Tanaka H, Wakamiya A, Murata Y, Suga S (2013) Synthesis of hexa(furan-2-yl)benzenes and their π-extended derivatives. J Org Chem 78(6):2763–2768
Mitsui C, Soeda J, Miwa K, Tsuji H, Takeya J, Nakamura E (2012) Naphtho[2,1-b:6,5-b’]difuran a versatile motif available for solution-processed single-crystal organic field-effect transistors with high hole mobility. J Am Chem Soc 134(12):5448–5451
Ponce Ortiz R, Herrera H, Mancheño MJ, Seoane C, Segura JL, Mayorga Burrezo P, Casado J, López Navarrete JT, Facchetti A, Marks TJ (2013) Molecular and electronic-structure basis of the ambipolar behavior of naphthalimide–terthiophene derivatives: implementation in organic field-effect transistors chemistry. A Eur J 19(37):12458–12467
Chaudhry AR, Ahmed R, Irfan A, Shaari A, Al-Sehemi AG (2013) Quantum chemical approach toward the electronic, photophysical and charge transfer properties of the materials used in organic field-effect transistors. Mater Chem Phys 138(2–3):468–478
Chaudhry AR, Ahmed R, Irfan A, Shaari A, Al-Sehemi AG (2014) Effects of electron withdrawing groups on transfer integrals, mobility, electronic and photo-physical properties of naphtho[2,1-b:6,5-b’]difuran derivatives: a theoretical study. Sci Adv Mater 6(8):1727–1739
Chaudhry AR, Ahmed R, Irfan A, Shaari A, Maarof H, Al-Sehemi AG (2014) First principles investigations of electronic, photoluminescence and charge transfer properties of the naphtho[2,1-b:6,5-b’]difuran and its derivatives for OFET. Sains Malays 43(6):867–875
Chaudhry AR, Ahmed R, Irfan A, Muhammad S, Shaari A, Al-Sehemi AG (2014) Effect of heteroatoms substitution on electronic, photophysical and charge transfer properties of naphtha [2,1-b:6,5-b’] difuran analogues by density functional theory. Comput Theor Chem 1045:123–134
Chaudhry AR, Ahmed R, Irfan A, Muhammad S, Shaari A, Al-Sehemi AG (2014) Influence of push-pull configuration on the electro-optical and charge transport properties of novel naphtho-difuran derivatives: a DFT study. RSC Adv 4(90):48876–48887
Becke AD (1993) Density-functional thermochemistry III. The role of exact exchange. J Chem Phys 98(7):5648–5652
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37(2):785–789
Hehre WJ, Ditchfield R, Pople JA (1972) Self-consistent molecular orbital methods XII. Further extensions of gaussian-type basis sets for Use in molecular orbital studies of organic molecules. J Chem Phys 56(5):2257–2261
Hariharan PC, Pople JA (1973) The influence of polarization functions on molecular orbital hydrogenation energies Theoretica. Chim Acta 28(3):213–222
Dill JD, Pople JA (1975) Self‐consistent molecular orbital methods. XV extended gaussian‐type basis sets for lithium, beryllium, and boron. J Chem Phys 62(7):2921–2923
Bauernschmitt R, Ahlrichs R (1996) Treatment of electronic excitations within the adiabatic approximation of time dependent density functional theory. Chem Phys Lett 256(4–5):454–464
Stratmann RE, Scuseria GE, Frisch MJ (1998) An efficient implementation of time-dependent density-functional theory for the calculation of excitation energies of large molecules. J Chem Phys 109(19):8218–8224
Van Caillie C, Amos RD (1999) Geometric derivatives of excitation energies using SCF and DFT. Chem Phys Lett 308(3–4):249–255
Van Caillie C, Amos RD (2000) Geometric derivatives of density functional theory excitation energies using gradient-corrected functionals. Chem Phys Lett 317(1–2):159–164
Furche F, Ahlrichs R (2002) Adiabatic time-dependent density functional methods for excited state properties. J Chem Phys 117(16):7433–7447
Casida ME, Jamorski C, Casida KC, Salahub DR (1998) Molecular excitation energies to high-lying bound states from time-dependent density-functional response theory:characterization and correction of the time-dependent local density approximation ionization threshold. J Chem Phys 108(11):4439–4449
Gruhn NE, da Silva Filho DA, Bill TG, Malagoli M, Coropceanu V, Kahn A, Brédas J-L (2002) The vibrational reorganization energy in pentacene: molecular influences on charge transport. J Am Chem Soc 124(27):7918–7919
Reimers JR (2001) A practical method for the use of curvilinear coordinates in calculations of normal-mode-projected displacements and duschinsky rotation matrices for large molecules. J Chem Phys 115(20):9103–9109
Irfan A, Cui R, Zhang J (2009) Fluorinated derivatives of mer-Alq3: energy decomposition analysis, optical properties, and charge transfer study. Theor Chem Acc 122(5–6):275–281
Coropceanu V, Nakano T, Gruhn NE, Kwon O, Yade T, K-i K, Brédas J-L (2006) Probing charge transport in π-stacked fluorene-based systems. J Phys Chem B 110(19):9482–9487
Li Y, Zou L-Y, Ren A-M, Feng J-K (2012) Theoretical study on the electronic structures and photophysical properties of a series of dithienylbenzothiazole derivatives. Comput Theor Chem 981:14–24
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09 Revision A.02. Gaussian Inc, Wallingford
Zhang Y, Cai X, Bian Y, Li X, Jiang J (2008) Heteroatom substitution of oligothienoacenes: from good p-type semiconductors to good ambipolar semiconductors for organic field-effect transistors. J Phys Chem C 112(13):5148–5159
Marcus RA (1993) Electron transfer reactions in chemistry theory and experiment. Rev Mod Phys 65(3):599–610
Brédas JL, Calbert JP, da Silva Filho DA, Cornil J (2002) Organic semiconductors: a theoretical characterization of the basic parameters governing charge transport. Proc Natl Acad Sci 99(9):5804–5809
Shinamura S, Osaka I, Miyazaki E, Nakao A, Yamagishi M, Takeya J, Takimiya K (2011) Linear- and angular-shaped naphthodithiophenes: selective synthesis properties, and application to organic field-effect transistors. J Am Chem Soc 133(13):5024–5035
Mohakud S, Alex AP, Pati SK (2010) Ambipolar charge transport in α-oligofurans: a theoretical study. J Phys Chem C 114(48):20436–20442
Yi Y, Zhu L, Brédas J-L (2012) Charge-transport parameters of acenedithiophene crystals: realization of one-, two-, or three-dimensional transport channels through alkyl and phenyl derivatizations. J Phys Chem C 116(8):5215–5224
Politzer P, Truhlar (Eds.) DG (1981) Chemical applications of atomic and molecular electrostatic potentials. Plenum, New York
Stewart RF (1979) On the mapping of electrostatic properties from bragg diffraction data. Chem Phys Lett 65(2):335–342
Murray JS, Politzer P (2011) The electrostatic potential: an overview. Mol Sci 1(2):153–163
Shkir M, Muhammad S, AlFaify S, Irfan A, Yahia IS (2015) A dual approach to study the electro-optical properties of a noncentrosymmetric l-asparagine monohydrate. Spectrochim Acta A Mol Biomol Spectrosc 137:432–441
Muhammad S, Xu H, Janjua MRSA, Su Z, Nadeem M (2010) Quantum chemical study of benzimidazole derivatives to tune the second-order nonlinear optical molecular switching by proton abstraction. Phys Chem Phys 12(18):4791–4799
Irfan A, Zhang J, Chang Y (2010) Theoretical investigations of the charge transfer properties of anthracene derivatives. Theor Chem Acc 127(5–6):587–594
Irfan A, Zhang J (2009) Effect of one ligand substitution on charge transfer and optical properties in mer-Alq3: a theoretical study. Theor Chem Acc 124(5–6):339–344
Acknowledgments
Authors are grateful to the Ministry of the Education/Universiti Teknologi Malaysia (UTM) for providing funding via project Q.J130000.2526.06H15 for the successful execution of this project and the King Khalid University (KKU) for providing the support and facilities to complete this research study.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Chaudhry, A.R., Ahmed, R., Irfan, A. et al. How does the increment of hetero-cyclic conjugated moieties affect electro-optical and charge transport properties of novel naphtha-difuran derivatives? A computational approach. J Mol Model 20, 2547 (2014). https://doi.org/10.1007/s00894-014-2547-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-014-2547-3