Abstract
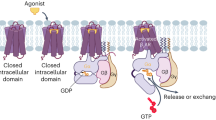
G-protein-coupled receptors (GPCRs) are currently one of the largest families of drug targets. The constitutive activation induced by mutation of key GPCR residues is associated closely with various diseases. However, the structural basis underlying such activation and its role in drug binding has remained unclear. Herein, we used all-atom molecular dynamics simulations and free energy calculations to study the effects of a D130N mutation on the structure of β2 adrenergic receptor (β2AR) and its binding of the agonist salbutamol. The results indicate that the mutation caused significant changes in some key helices. In particular, the mutation leads to the departure of transmembrane 3 (TM3) from transmembrane 6 (TM6) and marked changes in the NPxxY region as well as the complete disruption of a key ionic lock, all of which contribute to the observed constitutive activation. In addition, the D130N mutation weakens some important H-bonds, leading to structural changes in these regions. Binding free energy calculations indicate that van der Waals and electrostatic interactions are the main driving forces in binding salbutamol; however, binding strength in the mutant β2AR is significantly enhanced mainly through modifying electrostatic interactions. Further analysis revealed that the increase in binding energy upon mutation stems mainly from the H-bonds formed between the hydroxyl group of salbutamol and the serine residues of TM5. This observation suggests that modifications of the H-bond groups of this drug could significantly influence drug efficacy in the treatment of diseases associated with this mutation.

All-atom molecular dynamics simulation and free energy calculations were used to study the effects of the D130N mutation on the structure of β2 adrenergic receptor (β2AR) and its binding with salbutamol agonist. The results indicate that the mutation induces significant changes in some important regions and favors agonist binding mainly through increasing non-bond electrostatic interactions










Similar content being viewed by others
References
Takeda S, Kadowaki S, Haga T, Takaesu H, Mitaku S (2002) Identification of G protein-coupled receptor genes from the human genome sequence. FEBS Lett 520(1):97–101
Fredriksson R, Lagerström MC, Lundin L-G, Schiöth HB (2003) The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Mol Pharmacol 63(6):1256–1272
Congreve M, Langmead CJ, Mason JS, Marshall FH (2011) Progress in structure based drug design for G protein-coupled receptors. J Med Chem 54(13):4283–4311
Gether U (2000) Uncovering molecular mechanisms involved in activation of G protein-coupled receptors. Endocr Rev 21(1):90–113
Arvanitakis L, Geras-Raaka E, Gershengorn MC (1998) Constitutively signaling G-protein-coupled receptors and human disease. Trends Endocrinol Metab 9(1):27–31
Thompson MD, Burnham WM, Cole DE (2005) The G protein-coupled receptors: pharmacogenetics and disease. Crit Rev Clin Lab Sci 42(4):311–392
Dryja TP, McGee TL, Reichel E, Hahn LB, Cowley GS, Yandell DW, Sandberg MA, Berson EL (1990) A point mutation of the rhodopsin gene in one form of retinitis pigmentosa. Nature 343(6256):364–366
Rao VR, Cohen GB, Oprian DD (1994) Rhodopsin mutation G90D and a molecular mechanism for congenital night blindness. Nature 367(6464):639–642
Robinson PR, Cohen GB, Zhukovsky EA, Oprian DD (1992) Constitutively active mutants of rhodopsin. Neuron 9(4):719–725
Themmen APN, Martens JWM, Brunner HG (1997) Gonadotropin receptor mutations. J Endocrinol 153(2):179–183
Van Sande J, Parma J, Tonacchera M, Swillens S, Dumont J, Vassart G (1995) Somatic and germline mutations of the TSH receptor gene in thyroid diseases. J Clin Endocrinol Metab 80(9):2577–2585
Rasmussen SG, Choi HJ, Fung JJ, Pardon E, Casarosa P, Chae PS, DeVree BT, Rosenbaum DM, Thian FS, Kobilka TS (2011) Structure of a nanobody-stabilized active state of the β2 adrenoceptor. Nature 469(7329):175–180
Cherezov V, Rosenbaum DM, Hanson MA, Rasmussen SG, Thian FS, Kobilka TS, Choi HJ, Kuhn P, Weis WI, Kobilka BK et al (2007) High-resolution crystal structure of an engineered human β2-adrenergic G protein-coupled receptor. Science 318(5854):1258–1265
Park JH, Scheerer P, Hofmann KP, Choe HW, Ernst OP (2008) Crystal structure of the ligand-free G-protein-coupled receptor opsin. Nature 454(7201):183–187
Warne T, Serrano-Vega MJ, Baker JG, Moukhametzianov R, Edwards PC, Henderson R, Leslie AGW, Tate CG, Schertler GFX (2008) Structure of a β1-adrenergic G-protein-coupled receptor. Nature 454(7203):486–491
Rasmussen SGF, Jensen AD, Liapakis G, Ghanouni P, Javitch JA, Gether U (1999) Mutation of a highly conserved aspartic acid in the β2 adrenergic receptor: constitutive activation, structural instability, and conformational rearrangement of transmembrane segment 6. Mol Pharmacol 56(1):175–184
Probst WC, Snyder LA, Schuster DI, Brosius J, Sealfon SC (1992) Sequence alignment of the G-protein coupled receptor superfamily. DNA Cell Biol 11(1):1–20
Xue WW, Pan DB, Yang Y, Liu HX, Yao XJ (2012) Molecular modeling study on the resistance mechanism of HCV NS3/4A serine protease mutants R155K, A156V and D168A to TMC435. Antivir Res 93(1):126–137
Yang M-J, Pang XQ, Zhang X, Han KL (2011) Molecular dynamics simulation reveals preorganization of the chloroplast FtsY towards complex formation induced by GTP binding. J Struct Biol 173(1):57–66
Zhu LJ, Yang W, Meng YY, Xiao XC, Guo YZ, Pu XM, Li ML (2012) Effects of organic solvent and crystal water on γ-chymotrypsin in acetonitrile media: observations from molecular dynamics simulation and DFT calculation. J Phys Chem B 116(10):3292–3304
Li MH, Luo Q, Li ZS (2010) Molecular dynamics study on the interactions of porphyrin with two antiparallel human telomeric quadruplexes. J Phys Chem B 114(18):6216–6224
Li Z, Cai YH, Cheng YK, Lu X, Shao YX, Li XS, Liu M, Liu PQ, Luo H-B (2013) Identification of novel phosphodiesterase-4D inhibitors prescreened by molecular dynamics-augmented modeling and validated by bioassay. J Chem Inf Model 53(4):972–981
Vilar S, Karpiak J, Berk B, Costanzi S (2011) In silico analysis of the binding of agonists and blockers to the β2-adrenergic receptor. J Mol Graph Model 29(6):809–817
Johnston JM, Filizola M (2011) Showcasing modern molecular dynamics simulations of membrane proteins through G protein-coupled receptors. Curr Opin Struc Biol 21(4):552–558
Rubenstein RC, Wong S, Ross E (1987) The hydrophobic tryptic core of the beta-adrenergic receptor retains Gs regulatory activity in response to agonists and thiols. J Biol Chem 262(34):16655–16662
Guex N, Peitsch MC (1997) SWISS-MODEL and the Swiss-PdbViewer: an environment for comparative protein modeling. Electrophoresis 18(15):2714–2723
Filizola M, Wang SX, Weinstein H (2006) Dynamic models of G-protein coupled receptor dimers: indications of asymmetry in the rhodopsin dimer from molecular dynamics simulations in a POPC bilayer. J Comput Aided Mol Des 20(7–8):405–416
Duan Y, Wu C, Chowdhury S, Lee MC, Xiong GM, Zhang W, Yang R, Cieplak P, Luo R, Lee T (2003) A point-charge force field for molecular mechanics simulations of proteins based on condensed-phase quantum mechanical calculations. J Comput Chem 24(16):1999–2012
Jorgensen WL, Chandrasekhar J, Madura JD, Impey RW, Klein ML (1983) Comparison of simple potential functions for simulating liquid water. J Chem Phys 79(2):926–935
Wang JM, Wolf RM, Caldwell JW, Kollman PA, Case DA (2004) Development and testing of a general amber force field. J Comput Chem 25(9):1157–1174
Berendsen HJ, Postma JPM, van Gunsteren WF, DiNola A, Haak J (1984) Molecular dynamics with coupling to an external bath. J Chem Phys 81(8):3684–3690
Ryckaert J-P, Ciccotti G, Berendsen HJ (1977) Numerical integration of the cartesian equations of motion of a system with constraints: molecular dynamics of n-alkanes. J Comput Phys 23(3):327–341
York D, Darden T, Pedersen L, Anderson M (1993) Molecular dynamics simulation of HIV-1 protease in a crystalline environment and in solution. Biochemistry 32(6):1443–1453
Essmann U, Perera L, Berkowitz ML, Darden T, Lee H, Pedersen LG (1995) A smooth particle mesh Ewald method. J Chem Phys 103(19):8577–8593
Humphrey W, Dalke A, Schulten K (1996) VMD: visual molecular dynamics. J Mol Graph 14(1):33–38
Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, Olson AJ (2009) AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. J Comput Chem 30(16):2785–2791
Wang JM, Wang W, Kollman PA, Case DA (2006) Automatic atom type and bond type perception in molecular mechanical calculations. J Mol Graph Model 25(2):247–260
Miller BR III, McGee TD Jr, Swails JM, Homeyer N, Gohlke H, Roitberg AE (2012) MMPBSA.py: an efficient program for end-state free energy calculations. J Chem Theory Comput 8(9):3314–3321
Rastelli G, Degliesposti G, Del Rio A, Sgobba M (2009) Binding estimation after refinement, a new automated procedure for the refinement and rescoring of docked ligands in virtual screening. Chem Biol Drug Des 73(3):283–286
Lafont V, Armstrong AA, Ohtaka H, Kiso Y, Mario Amzel L, Freire E (2007) Compensating enthalpic and entropic changes hinder binding affinity optimization. Chem Biol Drug Des 69(6):413–422
Dror RO, Arlow DH, Maragakis P, Mildorf TJ, Pan AC, Xu HF, Borhani DW, Shaw DE (2011) Activation mechanism of the β2-adrenergic receptor. Proc Natl Acad Sci USA 108(46):18684–18689
Porter JE, Perez DM (1999) Characteristics for a salt-bridge switch mutation of the alpha(1b) adrenergic receptor—altered pharmacology and rescue of constitutive activity. J Biol Chem 274(49):34535–34538
Befort K, Zilliox C, Filliol D, Yue SY, Kieffer BL (1999) Constitutive activation of the δ opioid receptor by mutations in transmembrane domains III and VII. J Biol Chem 274(26):18574–18581
Huang P, Visiers I, Weinstein H, Liu-Chen L-Y (2002) The local environment at the cytoplasmic end of TM6 of the μ opioid receptor differs from those of rhodopsin and monoamine receptors: introduction of an ionic lock between the cytoplasmic ends of helices 3 and 6 by a L6. 30 (275) E mutation inactivates the μ opioid receptor and reduces the constitutive activity of its t6. 34 (279) k mutant. Biochemistry 41(40):11972–11980
Kim JM, Altenbach C, Kono M, Oprian DD, Hubbell WL, Khorana HG (2004) Structural origins of constitutive activation in rhodopsin: Role of the K296/E113 salt bridge. Proc Natl Acad Sci USA 101(34):12508–12513
Dror RO, Arlow DH, Borhani DW, Jensen MØ, Piana S, Shaw DE (2009) Identification of two distinct inactive conformations of the β2-adrenergic receptor reconciles structural and biochemical observations. Proc Natl Acad Sci USA 106(12):4689–4694
Vanni S, Neri M, Tavernelli I, Rothlisberger U (2010) A conserved protonation-induced switch can trigger “ionic-lock” formation in adrenergic receptors. J Mol Biol 397(5):1339–1349
Gether U, Lin S, Ghanouni P, Ballesteros JA, Weinstein H, Kobilka BK (1997) Agonists induce conformational changes in transmembrane domains III and VI of the β2 adrenoceptor. EMBO J 16(22):6737–6747
Wong CF, Kua J, Zhang Y, Straatsma TP, McCammon JA (2005) Molecular docking of balanol to dynamics snapshots of protein kinase A. Proteins 61(4):850–858
Bhattacharya S, Hall SE, Li H, Vaidehi N (2008) Ligand-stabilized conformational states of human β2 adrenergic receptor: insight into G-protein-coupled receptor activation. Biophys J 94(6):2027–2042
Ballesteros J, Kitanovic S, Guarnieri F, Davies P, Fromme BJ, Konvicka K, Chi L, Millar RP, Davidson JS, Weinstein H (1998) Functional microdomains in G-protein-coupled receptors the conserved arginine-cage motif in the gonadotropin-releasing hormone receptor. J Biol Chem 273(17):10445–10453
Barak LS, Tiberi M, Freedman NJ, Kwatra MM, Lefkowitz RJ, Caron MG (1994) A highly conserved tyrosine residue in G protein-coupled receptors is required for agonist-mediated beta 2-adrenergic receptor sequestration. J Biol Chem 269(4):2790–2795
Barak LS, Menard L, Ferguson SS, Colapietro A-M, Caron MG (1995) The conserved seven-transmembrane sequence NP (X) 2, 3Y of the G-protein-coupled receptor superfamily regulates multiple properties of the. beta. 2-adrenergic receptor. Biochemistry 34(47):15407–15414
Dixon R, Sigal I, Strader C (1988) Structure-function analysis of the β-adrenergic receptor. Cold Spring Harbor Symp Quant Biol 53:487–497
Gabilondo AM, Krasel C, Lohse MJ (1996) Mutations of Tyr326 in the β 2-adrenoceptor disrupt multiple receptor functions. Eur J Pharmacol 307(2):243–250
Simpson LM, Wall ID, Blaney FE, Reynolds CA (2011) Modeling GPCR active state conformations: the β2‐adrenergic receptor. Proteins 79(5):1441–1457
Warrell D, Robertson D, Howes JN, Conolly M, Paterson J, Beilin L, Dollery C (1970) Comparison of cardiorespiratory effects of isoprenaline and salbutamol in patients with bronchial asthma. BMJ 1(5688):65–70
Ekue JK, Shanks R, Zaidi S (1971) Comparison of the effects of isoprenaline, orciprenaline, salbutamol and isoetharine on the cardiovascular system of anaesthetized dogs. Br J Pharmacol 43(1):23–31
Fraser CM, Chung FZ, Wang CD, Venter JC (1988) Site-directed mutagenesis of human beta-adrenergic receptors: substitution of aspartic acid-130 by asparagine produces a receptor with high-affinity agonist binding that is uncoupled from adenylate cyclase. Proc Natl Acad Sci USA 85(15):5478–5482
Deng NJ, Cieplak P (2009) Insights into affinity and specificity in the complexes of α-lytic protease and its inhibitor proteins: binding free energy from molecular dynamics simulation. Phys Chem Chem Phys 11(25):4968–4981
Soriano-Ursúa MA, Trujillo-Ferrara JG, Correa-Basurto J, Vilar S (2013) Recent structural advances of β1 and β2 adrenoceptors yield keys for ligand recognition and drug design. J Med Chem 56(21):8207–8223
Liapakis G, Ballesteros JA, Papachristou S, Chan WC, Chen X, Javitch JA (2000) The forgotten serine a critical role for Ser-2035.42 in ligand binding to and Activation of the β2-adrenergic receptor. J Biol Chem 275(48):37779–37788
Sato T, Kobayashi H, Nagao T, Kurose H (1999) Ser203 as well as Ser204 and Ser207 in fifth transmembrane domain of the human β2‐adrenoceptor contributes to agonist binding and receptor activation. Brit J Pharmacol 128(2):272–274
Del Carmine R, Molinari P, Sbraccia M, Ambrosio C, Costa T (2004) “Induced-fit” mechanism for catecholamine binding to the β2-adrenergic receptor. Mol Pharmacol 66(2):356–363
Bhattacharya S, Vaidehi N (2010) Computational mapping of the conformational transitions in agonist selective pathways of a G-protein coupled receptor. J Am Chem Soc 132(14):5205–5214
Wieland K, Zuurmond HM, Krasel C, Ijzerman AP, Lohse MJ (1996) Involvement of Asn-293 in stereospecific agonist recognition and in activation of the beta 2-adrenergic receptor. Proc Natl Acad Sci USA 93(17):9276–9281
Hannawacker A, Krasel C, Lohse MJ (2002) Mutation of Asn293 to Asp in transmembrane helix VI abolishes agonist-induced but not constitutive activity of the β2-adrenergic receptor. Mol Pharmacol 62(6):1431–1437
Acknowledgment
This project was supported by the National Science Foundation of China (Grant No. 21273154, U1230121).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 22 kb)
Rights and permissions
About this article
Cite this article
Zhu, Y., Yuan, Y., Xiao, X. et al. Understanding the effects on constitutive activation and drug binding of a D130N mutation in the β2 adrenergic receptor via molecular dynamics simulation. J Mol Model 20, 2491 (2014). https://doi.org/10.1007/s00894-014-2491-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-014-2491-2