Abstract
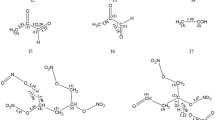
To improve the understanding of the unimolecular decomposition mechanism of nitroglycerin (NG) in the gas phase, density functional theory calculations were performed to determine various decomposition channels at the B3LYP/6-311G** level. For the unimolecular decomposition mechanism of NG, we find two main mechanisms: (I) homolytic cleavage of O-NO2 to form •NO2 and CH2ONO2CHONO2CH2O•, which subsequently decomposes to form •CHO, •NO2, and 2CH2O; (II) successive HONO eliminations to form HONO and CHO-CO-CHO, which subsequently decomposes to form CH2O + 2CO2 and •CHO + CO. We also find that the former channel has slightly smaller activation energy than the latter one. In addition, the rate constants of the initial process of the two decomposition channels were calculated. The results show that the O-NO2 cleavage pathway occurs more easily than the HONO elimination.





Similar content being viewed by others
References
Sadasivan N, Bhaumik A (1984) J Therm Anal 29:1043–1052
Li SN, Liu Y, Tuo XL, Wang XG (2008) Polymer 49:2775–2780
McDonald BA (2011) Propell Explos Pyrot 36:576–583
Yi JH, Zhao FQ, Xu SY, Gao HX, Hu RZ (2008) Chem Res Chinese U 24:608–614
Sui X, Wang NF, Wan QA, Bi SH (2010) Propell Explos Pyrot 35:535–539
Zhao YJ, Zhang W, Zhang XG, Zhu H, Wang CH, Fang LJ (2007) Theory Pract Energ Mater 7:163–166
Zhang TH (2002) J Energ Mater 20:175–189
Hiegel GA, Nguyen J, Zhou Y (2004) Synth Commun 34:2507–2511
Chas EW, Krastins G (1970) J Phys Chem 74:999–1006
Roos BD, Brill TB (2002) Combust Flame 128:181–190
Hiyoshi RI, Brill TB (2002) Propell Explos Pyrot 27:23–30
Chin A, Ellison DS, Poehlein SK (2007) Propell Explos Pyrot 32:117–126
Roos BD, Brill TB (2001) Propell Explos Pyrot 26:213–220
Tompa AS (1980) J Hazard Mater 4:95–112
Sućeska M, Mušanić SM, Houra IF (2010) Thermochim Acta 510:9–16
Toland A, Simmie JM (2003) Combust Flame 132:556–564
Devyatykh GG, Zaslonko IS, Smirnov VN, Moiseev AN, Votintsev VN, Tereza AM (1993) Kinet Catal 34:185–189
Oxley JC, Smith JL, Rogers E, Ye W, Aradi AA, Henly TJ (2000) Energ Fuel 14:1252–1264
Correra TC, Riveros JM (2010) J Phys Chem A 114:11910–11919
Makashir PS, Mahajan RR, Agrawal JP (1995) J Therm Anal 45:501–509
Michael AH, Kay RB, Jimmie CO (1991) J Phys Chem 95:3955–3960
Francisco MA, Krylowski J (2005) Ind Eng Chem Res 44:5439–5446
Gong XD, Xiao HM (2000) J Mol Struc (Theochem) 498:181–190
Gong XD, Xiao HM (2001) J Mol Struc (Theochem) 572:213–221
Wang DX, Xiao HM (1992) J Phys Org Chem 5:361–366
Tan JZ, Xiao HM, Gong XD, Li JS (2001) Chinese J Chem 19:931–937
Li MM, Wang GX, Guo XD, Wu ZW, Song HC (2009) J Mol Struc (Theochem) 900:90–95
Yonei T, Hashimoto K, Arai M, Tamura M (2003) Energ Fuel 17:725–730
Xiao HM, Fan JF, Li YF (1990) Chinese J Chem 8:390–395
Becke AD (1993) J Chem Phys 98:5648–5652
Lee C, Yang W, Parr RG (1988) Phys Rev B 37:785–789
Gonzalez C, Schlegel HB (1989) J Chem Phys 90:2154
Gonzalez C, Schlegel HB (1990) J Phys Chem 94:5523
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA Jr, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2003) Gaussian 03. Gaussian, Inc., Pittsburgh, PA
Luo YR (2003) Handbook of bond dissociation energies in organic compouds. CRC Press, Washington, DC
Chakraborty D, Muller RP, Dasgupta S, Goddard WA (2001) J Phys Chem A 105:1302–1314
Chakraborty D, Muller RP, Dasgupta S, Goddard WA (2000) J Phys Chem A 104:2261–2272
Acknowledgments
This work was supported by the National “973” Project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yan, Q., Zhu, W., Pang, A. et al. Theoretical studies on the unimolecular decomposition of nitroglycerin. J Mol Model 19, 1617–1626 (2013). https://doi.org/10.1007/s00894-012-1724-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-012-1724-5